Payload Information
General Information of This Payload
| Payload ID | PAY0HZXQT |
|||||
|---|---|---|---|---|---|---|
| Name | Idarubicin |
|||||
| Synonyms |
IDARUBICIN; 58957-92-9; 4-Demethoxydaunorubicin; 4-Demethoxydaunomycin; Idarubicin Hcl; Idarubicina; Idarubicine; Idamycin; Idarubicine [INN-French]; Idarubicinum [INN-Latin]; Idarubicina [INN-Spanish]; Idarubicinum; Idarubicin [INN:BAN]; IMI-30; 4-Desmethoxydaunorubicin; Daunomycin, 4-demethoxy-; Idarubicin (INN); Zavedos (TN); CCRIS 5083; NSC 256439; UNII-ZRP63D75JW; 4-DMD; ZRP63D75JW; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; CHEBI:42068; NSC-256439; demethoxydaunorubicin; 5,12-Naphthacenedione, 9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)-; Idamycin (TN); IDARUBICIN [INN]; Idarubicin Hcl Pfs; I 1656; Idarubicinhydrochloride; (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,9,11-trihydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione; DM5; MLS001401448; NSC256439; (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-; NCGC00093976-03; SMR000466355; SR-01000075934; C26-H27-N-O9; DMDR; IDARUBICIN [MI]; IDARUBICIN [VANDF]; D01XDL; SCHEMBL3750; CHEMBL1117; IDARUBICIN [WHO-DD]; Lopac0_000600; KBioSS_002388; Idarubicin hydrochloride, solid; cid_636362; GTPL7083; 4-DEMETHOXY-DAUNORUBICIN; DTXSID7023142; BDBM58490; XDXDZDZNSLXDNA-TZNDIEGXSA-N; BCPP000207; HMS2089D05; HMS3261H22; Tox21_500600; HY-17381A; AKOS015895563; AC-9384; BCP9000773; CCG-204689; DB01177; LP00600; SDCCGSBI-0050582.P002; NCGC00093976-01; NCGC00093976-02; NCGC00093976-04; NCGC00093976-05; NCGC00093976-18; NCGC00261285-01; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; LS-94015; CS-0007534; EU-0100600; D08062; AB00698511-06; AB00698511-08; AB00698511-09; AB00698511-10; AB00698511_11; EN300-7479233; A832088; A935911; Q1063862; SR-01000075934-1; BRD-K69650333-001-01-1; BRD-K69650333-001-02-9; BRD-K69650333-003-14-0; Idarubicin, United States Pharmacopeia (USP) Reference Standard; (1S,3S)-3-ACETYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-6,11-DIOXO-1-NAPHTHACENYL 3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSIDE; (1s,3s)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-A-l-lyxo-hexopyranoside; (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-7-[(2R,4S,5S,6S)-4-azanyl-6-methyl-5-oxidanyl-oxan-2-yl]oxy-9-ethanoyl-6,9,11-tris(oxidanyl)-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; (7S,9S)-9-Acetyl-7-(((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (7S,9S)-9-acetyl-7-((2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-quinone;hydrochloride; (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-9-acetyl-7-[[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-2-oxanyl]oxy]-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione;hydrochloride; (7S-cis)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-9-acetyl-7-((3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy)-6,9,11-trihydroxy-, (7S-cis)-; 5,12-NAPHTHACENEDIONE, 9-ACETYL-7-((3-AMINO-2,3,6-TRIDEOXY-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-7,8,9,10-TETRAHYDRO-6,9,11-TRIHYDROXY-, (7S,9S)-; 5,12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-.alpha.-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-, (7S-cis)-
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 2-alpha (TOP2A) | |||||
| Structure |
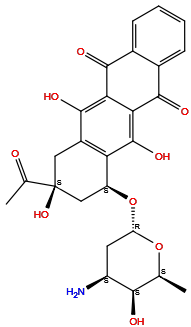
|
|||||
| Formula | C26H27NO9 |
|||||
| Isosmiles | C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=CC=CC=C5C4=O)O)(C(=O)C)O)N)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1
|
|||||
| InChIKey |
XDXDZDZNSLXDNA-TZNDIEGXSA-N
|
|||||
| IUPAC Name |
(7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
497.5 |
Polar area |
177 |
||
Complexity |
912 |
xlogp Value |
1.9 |
|||
Heavy Count |
36 |
Rot Bonds |
3 |
|||
Hbond acc |
10 |
Hbond Donor |
5 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 12 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 12300 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 15 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 2300 | nM |
DA-3 cells
|
Mouse lymphoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 3200 | nM |
ES2 cells
|
Ewing sarcoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | >33000 | nM |
Vero C1008 cells
|
Normal
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | >33000 | nM |
Vero C1008 cells
|
Normal
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 4500 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 6620 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 7300 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[5] |
Each Antibody-drug Conjugate Related to This Payload
References
