Payload Information
General Information of This Payload
| Payload ID | PAY0HAXGX |
|||||
|---|---|---|---|---|---|---|
| Name | Docetaxel |
|||||
| Synonyms |
Docetaxel; 114977-28-5; Taxotere; Docetaxel anhydrous; Docetaxol; Docetaxel Winthrop; EmDOC; RP-56976; Docetaxolum; Docefrez; Taxoel; Docetaxel Kabi; Docetaxel [INN]; NSC 628503; Docetaxel, Trihydrate; N-debenzoyl-N-tert-butoxycarbonyl-10-deacetyltaxol; RP 56976; taxotel; Docecad; DTXSID0040464; Docetaxel Teva; Docetaxel Accord; Taxotere(R); TXL; Taxotere (TN); Docetaxel, anhydrous; NSC-628503; UNII-699121PHCA; CHEBI:4672; DOCETAXEL MYLAN; Docetaxel hydrate; HSDB 6965; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol; 699121PHCA; Docetaxolum [INN-Latin]; DOCETAXEL TEVA PHARMA; docetere; N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetylpaclitaxel; docetaxel 114977-28-5; DTXCID8020464; XRP-6976L; ANX-514; SDP-014; SID 530; Docetaxel (INN); NSC628503; N-debenzoyl-N-Boc-10-deacetyl taxol; CABAZITAXEL METABOLITE (RP56976); [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-acetyloxy-1,9,12-trihydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate; Benzenepropanoic acid, beta-(((1,1-dimethylethoxy)carbonyl)amino)-alpha-hydroxy-, 12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester; DOCETAXEL (USP-RS); UNII-15H5577CQD; Docetaxel intermediate; BIND 014; DOCETAXEL (EP MONOGRAPH); DOCETAXEL (USP IMPURITY); CKD-810; DOCETAXEL (USP MONOGRAPH); (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1,9,12-trihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-2-yl benzoate; (2alpha,5beta,7beta,10beta,13alpha)-4-(acetyloxy)-13-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1,7,10-trihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate; 4-(acetyloxy)-13alpha-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1,7beta,10beta-trihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate; Benzenepropanoic acid, beta-(((1,1-dimethylethoxy)carbonyl)amino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (alphaR,betaS)-; CAS-114977-28-5; NSC 759850; DOCETAXEL TRIHYDRATE (EP MONOGRAPH); docetaxelum; C43H53NO14; MFCD00871399; Docetaxel (TN); Docetaxel- Bio-X; NCGC00181306-01; NCGC00181306-02; 4-(acetyloxy)-13alpha-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-1,7beta,10beta-trihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate; Taxotere (Aventis); CID148124; Docetaxolum (Latin); Docetaxel - Taxotere; RP56976; bind-014; Docetaxel (JAN/INN); DOCETAXEL [JAN]; DOCETAXEL [MI]; CHEMBL92; DOCETAXEL [HSDB]; Benzenepropanoic acid, beta-(((1,1-dimethylethoxy)carbonyl)amino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,1313-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, trihydrate, (alphaR,betaS)-; D0O5WP; Taxotere/Docetaxel Winthrop; SCHEMBL4419; N Debenzoyl N tert butoxycarbonyl 10 deacetyltaxol; GTPL6809; BIND 014 [WHO-DD]; BDBM36351; SYP-0704A; ZDZOTLJHXYCWBA-VCVYQWHSSA-; AMY4356; L01CD02; ZDZOTLJHXYCWBA-VCVYQWHSSA-N; 114977-28-5, Docetaxel; HMS2089K08; EX-A1206; HY-B0011; Tox21_112781; Tox21_113088; AC-383; DOCETAXEL ANHYDROUS [WHO-DD]; AKOS015960718; AKOS024457953; C43-H53-N-O14; Tox21_112781_1; CS-1144; CS-O-11300; DB01248; KS-1452; Docetaxel, purum, >=97.0% (HPLC); NCGC00181306-04; NCGC00242509-01; BD164373; D4102; D07866; EN300-123047; AB01273941-01; AB01273941-02; Q420436; SR-01000003023; W-60384; Q-100074; SR-01000003023-5; BRD-K30577245-001-04-3; BRD-K30577245-341-01-9; Z1546621742; Anhydrous Docetaxel, European Pharmacopoeia (EP) Reference Standard; (1S,2S,3R,4S,5R,7S,8S,10R,13S)-4-Acetoxy-2-benzoyloxy-5,20-epoxy-1,7,10-trihydroxy-9-oxotax-11-en-13-yl (2R,3S)-3-(1,1-dimethylethyl)oxycarbonylamino-2-hydroxy-3-phenylpropanoate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (aR,bS)-b-[[(1,1-dimethylethoxy)carbonyl]amino]-a-hydroxybenzenepropanoate; (2R,3S)-N-CARBOXY-3-PHENYLISOSERINE, N-TERT-BUTYL ESTER, 13-ESTER WITH 5.BETA.,20-EPOXY-1,2.ALPHA.,4,7.BETA.,10.BETA.,13.ALPHA.-HEXAHYDROXYTAX-11-EN-9-ONE 4-ACETATE 2-BENZOATE; (2R,3S)-N-CARBOXY-3-PHENYLISOSERINE, N-TERT-BUTYL ESTER, 13-ESTER WITH 5beta,20-EPOXY-1,2alpha,4,7beta,10beta,13alpha-HEXAHYDROXYTAX-11-EN-9-ONE 4-ACETATE 2-BENZOATE; [2aR-[2a?,4?,4a?,6?,9?(?R*,?S*),11?,12?,12a?,12b?]]-?-[[(1,1-Dimethylethoxy)carbonyl]amino]-?-hydroxy-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester benzenepropanoic acid; [acetoxy-[(2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenyl-propanoyl]oxy-trihydroxy-tetramethyl-oxo-[?]yl] benzoate; 114915-20-7; Benzenepropanoic acid, beta-(((1,1-dimethylethoxy)carbonyl)amino)-alpha-hydroxy-, (2aR,4S,4aR,6R,9S,11S,12S,12aS,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,4a,6,11-tetrahydroxy-8,12a,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (alphaR)-, hydrate (1:3); Benzenepropanoic acid,1-dimethylethoxy)carbonyl]amino]-.alpha.-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (.alpha.R,.beta.S)
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
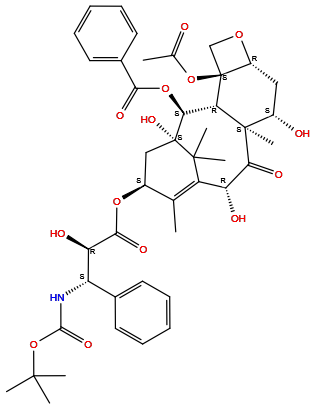
|
|||||
| Formula | C43H53NO14 |
|||||
| Isosmiles | [H]O[C@@]1([H])C(=O)[C@@]2(C([H])([H])[H])[C@]([H])([C@]([H])(OC(=O)c3c([H])c([H])c([H])c([H])c3[H])[C@@]3(O[H])C(C([H])([H])[H])(C([H])([H])[H])C1=C(C([H])([H])[H])[C@@]([H])(OC(=O)[C@]([H])(O[H])[C@]([H])(c1c([H])c([H])c([H])c([H])c1[H])N([H])C(=O)OC(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])C3([H])[H])[C@]1(OC(=O)C([H])([H])[H])C([H])([H])O[C@]1([H])C([H])([H])[C@]2([H])O[H] |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41+,42-,43+/m0/s1
|
|||||
| InChIKey |
ZDZOTLJHXYCWBA-VCVYQWHSSA-N
|
|||||
| IUPAC Name |
[(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-acetyloxy-1,9,12-trihydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
807.89 |
Polar area |
224.45 |
||
Complexity |
807.3466054 |
xlogp Value |
3.2596 |
|||
Heavy Count |
58 |
Rot Bonds |
17 |
|||
Hbond acc |
14 |
Hbond Donor |
5 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.006 | ug/mL |
22RV1 cells
|
Prostate carcinoma
|
[1] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.009 | ug/mL |
PC-3 cells
|
Prostate carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | ug/mL |
DU145 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.1 | nM |
DU145 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.1 | nM |
DU145 cells
|
Prostate carcinoma
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.13 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.2 | nM |
LNCaP cells
|
Prostate carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.2 | nM |
LNCaP cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.3 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.4 | nM |
Calu-1 cells
|
Lung squamous cell carcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.5 | nM |
PC-3 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.5 | nM |
PC-3 cells
|
Prostate carcinoma
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.5 | nM |
MV4-11 cells
|
Childhood acute monocytic leukemia
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.5 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.6 | nM |
PC-3 cells
|
Prostate carcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.6 | nM |
PC-3 cells
|
Prostate carcinoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.7 | nM |
U-251MG cells
|
Astrocytoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.75 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.2 | nM |
DU145 cells
|
Prostate carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.2 | nM |
DU145 cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.7 | nM |
HCT 116 cells
|
Colon carcinoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.7 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.8 | nM |
SH-SY5Y cells
|
Bone marrow neuroblastoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.9 | nM |
PC-3 cells
|
Prostate carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.9 | nM |
PC-3 cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
DU145 cells
|
Prostate carcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[15] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 10.3 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 11.2 | nM |
A2780 cells
|
Ovarian endometrioid adenocarcinoma
|
[17] | |
| Half Maximal Inhibitory Concentration (IC50) | 11.8 | nM |
MM1.S cells
|
Plasma cell myeloma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 110 | nM |
HEK293 cells
|
Normal
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 116540 | nM |
CCD-18Co cells
|
Normal
|
[18] | |
| Half Maximal Inhibitory Concentration (IC50) | 12.5 | nM |
DND-41 cells
|
T acute lymphoblastic leukemia
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 127 | nM |
P388 cells
|
Lymphoma
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 129.8 | nM |
HCT 116 cells
|
Colon carcinoma
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | 13 | nM |
PC-3 cells
|
Prostate carcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 130 | nM |
Vero cells
|
Normal
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 13000 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[22] | |
| Half Maximal Inhibitory Concentration (IC50) | 14.2 | nM |
Z-138 cells
|
Mantle cell lymphoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 15.2 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 1530.5 | nM |
NCI-ADR-RES cells
|
High grade ovarian serous adenocarcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 16 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 1680 | nM |
L132 cells
|
Endocervical adenocarcinoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 17.5 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 1730 | nM |
RWPE-1 cells
|
Normal
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 19 | nM |
Capan-1 cells
|
Pancreatic ductal adenocarcinoma
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 19.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 1940 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 19700 | nM |
HEK293 cells
|
Normal
|
[18] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
LN-229 cells
|
Glioblastoma
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[19] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 2 | nM |
SK-MEL19 cells
|
Melanoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.4 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.7 | nM |
T84 cells
|
Colon adenocarcinoma
|
[28] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[18] | |
| Half Maximal Inhibitory Concentration (IC50) | 210 | nM |
WPMY-1 cells
|
Normal
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 22 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 2340 | nM |
PC-3 cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 25 | nM |
Capan-1 cells
|
Pancreatic ductal adenocarcinoma
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 25000 | nM |
TERT-RPE1 cells
|
Normal
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 26 | nM |
DU145 cells
|
Prostate carcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 27.9 | nM |
PC3/DX cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 2860 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.5 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[17] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.5 | nM |
HCT 116 cells
|
Colon carcinoma
|
[18] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.55 | nM |
A2780-1A9 cells
|
Ovarian adenocarcinoma
|
[29] | |
| Half Maximal Inhibitory Concentration (IC50) | 3000 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 3000 | nM |
Z-138 cells
|
Mantle cell lymphoma
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 32 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 3230 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 3410 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 352.3 | nM |
DU145-TxR cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 3600 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 38 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 39 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 4 | nM |
HCT 116 cells
|
Colon carcinoma
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 4 | nM |
HCT 116 cells
|
Colon carcinoma
|
[28] | |
| Half Maximal Inhibitory Concentration (IC50) | 4 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 4 | nM |
Z-138 cells
|
Mantle cell lymphoma
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.7 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[28] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.95 | nM |
DU145 cells
|
Prostate carcinoma
|
[30] | |
| Half Maximal Inhibitory Concentration (IC50) | 4000 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[22] | |
| Half Maximal Inhibitory Concentration (IC50) | 410 | nM |
NCI-ADR-RES cells
|
High grade ovarian serous adenocarcinoma
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 43.25 | nM |
DU145 cells
|
Prostate carcinoma
|
[30] | |
| Half Maximal Inhibitory Concentration (IC50) | 47 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
PC-3 cells
|
Prostate carcinoma
|
[31] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
DU145 cells
|
Prostate carcinoma
|
[32] | |
| Half Maximal Inhibitory Concentration (IC50) | 50 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 50 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 55.3 | nM |
TERT-RPE1 cells
|
Normal
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 60 | nM |
HEp-2 cells
|
Laryngeal carcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 61 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 680 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 7 | nM |
HCT 116 cells
|
Colon carcinoma
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 7 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 7 | nM |
P388 cells
|
Lymphoma
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 7.2 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 7.3 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 70 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 70 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 734 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 8 | nM |
PC-3 cells
|
Prostate carcinoma
|
[31] | |
| Half Maximal Inhibitory Concentration (IC50) | 8.8 | nM |
Capan-1 cells
|
Pancreatic ductal adenocarcinoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[33] | |
| Half Maximal Inhibitory Concentration (IC50) | 8300 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[34] | |
| Half Maximal Inhibitory Concentration (IC50) | 836.2 | nM |
A549/TR cells
|
Lung adenocarcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 8580 | nM |
DU145 cells
|
Prostate carcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 860 | nM |
HEY cells
|
Ovarian carcinoma
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 890 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 9 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 9 | nM |
Z-138 cells
|
Mantle cell lymphoma
|
[23] | |
| Half Maximal Inhibitory Concentration (IC50) | 9.8 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 90 | nM |
SiHa cells
|
Cervical squamous cell carcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 950 | nM |
Capan-1 cells
|
Pancreatic ductal adenocarcinoma
|
[26] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
MM-310 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [35] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT03076372 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase-1 study evaluating the safety, pharmacology and preliminary activity of MM-310 in patients with solid tumors.
|
||||
Docetaxel-trastuzumab [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [36] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.00% (Day 67) | Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
Docetaxel and trastuzumab (5 and 1.9 mg/kg, respectively,every seven days 3) induces efficient tumor cell killing in cell line-derived models of SKBR3 cells with HER2 expression with high expression.
|
||||
| In Vivo Model | SKBR-3 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
References
