Payload Information
General Information of This Payload
| Payload ID | PAY0FQNWH |
|||||
|---|---|---|---|---|---|---|
| Name | DC1 |
|||||
| Synonyms |
169901-27-3; DC1; (S)-N-(2-(1-(Chloromethyl)-5-hydroxy-2,3-dihydro-1H-benzo[e]indole-3-carbonyl)-1H-indol-5-yl)-5-(3-mercaptopropanamido)-1H-indole-2-carboxamide; SCHEMBL12126075; AKOS040732933; HY-112899; CS-0067645; N-{2-[(1S)-1-(chloromethyl)-5-hydroxy-1H,2H,3H-benzo[e]indole-3-carbonyl]-1H-indol-5-yl}-5-(3-sulfanylpropanamido)-1H-indole-2-carboxamide
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
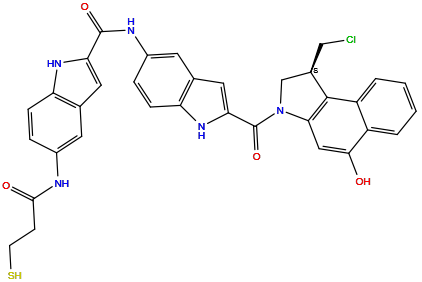
|
|||||
| Formula | C34H28ClN5O4S |
|||||
| Isosmiles | C1[C@H](C2=C(N1C(=O)C3=CC4=C(N3)C=CC(=C4)NC(=O)C5=CC6=C(N5)C=CC(=C6)NC(=O)CCS)C=C(C7=CC=CC=C72)O)CCl |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C34H28ClN5O4S/c35-16-20-17-40(29-15-30(41)23-3-1-2-4-24(23)32(20)29)34(44)28-14-19-12-22(6-8-26(19)39-28)37-33(43)27-13-18-11-21(5-7-25(18)38-27)36-31(42)9-10-45/h1-8,11-15,20,38-39,41,45H,9-10,16-17H2,(H,36,42)(H,37,43)/t20-/m1/s1
|
|||||
| InChIKey |
ZASLXALGERLDLT-HXUWFJFHSA-N
|
|||||
| IUPAC Name |
N-[2-[(1S)-1-(chloromethyl)-5-hydroxy-1,2-dihydrobenzo[e]indole-3-carbonyl]-1H-indol-5-yl]-5-(3-sulfanylpropanoylamino)-1H-indole-2-carboxamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
638.1 |
Polar area |
131 |
||
Complexity |
1120 |
xlogp Value |
5.4 |
|||
Heavy Count |
45 |
Rot Bonds |
7 |
|||
Hbond acc |
5 |
Hbond Donor |
6 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
B4-SPP-DC1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.40 pM
|
Positive CD19 expression (CD19+++/++) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.30 nM
|
Negative CD19 expression(CD19-) | ||
| Method Description |
Cytotoxicity of B4-SPP-DCx Conjugates (without acid phosphatase treatment) against Ramos (Ag+) and HL60/s (Ag-) Cells.
|
||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
References
