Payload Information
General Information of This Payload
| Payload ID | PAY0ETAAN |
|||||
|---|---|---|---|---|---|---|
| Name | Cabazitaxel |
|||||
| Synonyms |
CABAZITAXEL; 183133-96-2; Jevtana; Taxoid XRP6258; Xrp6258; XRP-6258; Cabazitaxelum; TXD 258; Jevtana Kit; Jevtana (TN); cabazitaxel acetonate; Cabazitaxel Injection; TXD258; XRP 6258; Cabazitaxel [USAN:INN]; NSC-761432; javtana; CHEBI:63584; TXD-258; UNII-51F690397J; RPR 116258A; RPR-116258A; 51F690397J; dimethoxydocetaxel; kabazitaxel; Cabazitaxel (Jevtana); (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-2-yl benzoate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate.; CABAZITAXEL ACETONATE [JAN]; Jevanta; XRP6258;RPR-116258A;taxoid XRP6258; CABAZITAXEL [MI]; CABAZITAXEL [INN]; Cabazitaxel (USAN/INN); CABAZITAXEL [USAN]; D02HSB; CABAZITAXEL [VANDF]; CABAZITAXEL [MART.]; CABAZITAXEL [WHO-DD]; SCHEMBL179674; CABAZITAXEL [EMA EPAR]; GTPL6798; CHEMBL1201748; AMY4317; CABAZITAXEL [ORANGE BOOK]; DTXSID40171389; EX-A838; BMQGVNUXMIRLCK-OAGWZNDDSA-N; (alpha-R,-beta-S)-beta-[[(1,1-Dimethylethoxy)carbonyl]amino]-alpha-hydroxybenzenepropanoic acid (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester; MFCD18827611; NSC761432; NSC794609; s3022; AKOS032947285; CCG-270519; CS-0972; CS-O-01257; DB06772; NSC 761432; NSC-794609; NCGC00346704-01; NCGC00346704-03; AS-75355; HY-15459; C3390; A25044; D09755; AB01273971-01; AB01273971_02; EN300-22232477; Q412963; SR-01000941585; J-011721; J-519981; SR-01000941585-1; (((tertbutoxy)carbonyl)amino)-2-hydroxy-3-phenylpropanoate1-hydroxy-7beta,10beta-dimethoxy-9-oxo-5beta,20-epoxytax-11-ene-2alpha,4,13alpha-triyl 4-acetate 2-benzoate 13-((2R,3S)-3-; (1S)-5beta,20-Epoxy-9-oxo-7beta,10beta-dimethoxytaxa-11-ene-1,2alpha,4alpha,13alpha-tetraol 2-benzoate 4-acetate 13-[(2R,3S)-2-hydroxy-3-(tert-butoxycarbonylamino)-3-phenylpropionate]; (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0(3),(1)?.0?,?]heptadec-13-en-2-yl benzoate; (2alpha,5beta,7beta,10beta,13alpha)-4-acetoxy-13-({(2R,3S)-3-[(tert-butoxycarbonyl)amino]-2-hydroxy-3-phenylpropanoyl}oxy)-1-hydroxy-7,10-dimethoxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate; (2AR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,1; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-3-phenylpropanoyl)oxy)-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate; [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-acetyloxy-1-hydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate; 1-hydroxy-7,10-dimethoxy-9-oxo-5,20-epoxytax-11-ene-2,4,13-triyl 4-acetate 2-benzoate 13-((2R,3S)-3-(((tertbutoxy)carbonyl)amino)-2-hydroxy-3-phenylpropanoate); 1-HYDROXY-7.BETA.,10.BETA.-DIMETHOXY-9-OXO-5.BETA.,20-EPOXYTAX-11-ENE-2.ALPHA.,4,13.ALPHA.-TRIYL 4-ACETATE 2-BENZOATE 13-((2R,3S)-3-(((TERT-BUTOXY)CARBONYL)AMINO)-2-HYDROXY-3-PHENYLPROPANOATE); 890654-44-1; Benzenepropanoic acid, beta-[[(1,1-dimethylethoxy)carbonyl]amino]-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4,6-dimethoxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (alphaR,betaS)-
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
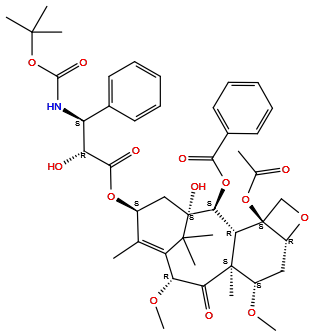
|
|||||
| Formula | C45H57NO14 |
|||||
| Isosmiles | CC1=C2[C@H](C(=O)[C@@]3([C@H](C[C@@H]4[C@]([C@H]3[C@@H]([C@@](C2(C)C)(C[C@@H]1OC(=O)[C@@H]([C@H](C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)OC)C)OC |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,32-,33+,34+,35-,37-,43+,44-,45+/m0/s1
|
|||||
| InChIKey |
BMQGVNUXMIRLCK-OAGWZNDDSA-N
|
|||||
| IUPAC Name |
[(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-acetyloxy-1-hydroxy-15-[(2R,3S)-2-hydroxy-3-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]oxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
835.9 |
Polar area |
202 |
||
Complexity |
1690 |
xlogp Value |
2.7 |
|||
Heavy Count |
60 |
Rot Bonds |
15 |
|||
Hbond acc |
14 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.26 | nM |
NCI-H524 cells
|
Lung small cell carcinoma
|
[1] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.3553 | nM |
SGC-7901 cells
|
Gastric carcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 0.5391 | nM |
U-937 cells
|
Adult acute monocytic leukemia
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.187 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.283 | nM |
PANC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.406 | nM |
HT-1080 cells (FAP expression)
|
Fibrosarcoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.429 | nM |
DU145 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.48 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.483 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.483 | nM |
A-431 cells
|
Skin squamous cell carcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 1.799 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 15 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 15 | nM |
MES-SA/Dx5 cells
|
Uterine sarcoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 4.186 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 4.736 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 467.2 | nM |
BGC-823 cells
|
Stomach adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 5000 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 62.5 | nM |
A549/TR cells
|
Lung adenocarcinoma
|
[4] |
Each Antibody-drug Conjugate Related to This Payload
References
