Payload Information
General Information of This Payload
| Payload ID | PAY0DWOSZ |
|||||
|---|---|---|---|---|---|---|
| Name | Tiancimycin |
|||||
| Synonyms |
Tiancimycin; CHEMBL4636420; SCHEMBL17918234
Click to Show/Hide
|
|||||
| Target(s) | Microtubule (MT) | |||||
| Structure |
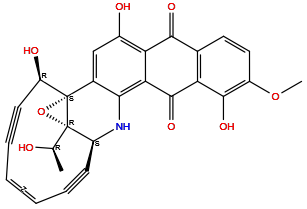
|
|||||
| Formula | C27H19NO8 |
|||||
| Isosmiles | C[C@H]([C@]12[C@@H]3C#C/C=C\C#C[C@H]([C@@]1(O2)C4=CC(=C5C(=C4N3)C(=O)C6=C(C5=O)C=CC(=C6O)OC)O)O)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C27H19NO8/c1-12(29)26-17-7-5-3-4-6-8-18(31)27(26,36-26)14-11-15(30)20-21(22(14)28-17)25(34)19-13(23(20)32)9-10-16(35-2)24(19)33/h3-4,9-12,17-18,28-31,33H,1-2H3/b4-3-/t12-,17+,18-,26+,27+/m1/s1
|
|||||
| InChIKey |
DJOMZYYFFHCVSQ-UBZKRVARSA-N
|
|||||
| IUPAC Name |
(1S,17S,20Z,24R,26R)-4,11,24-trihydroxy-26-[(1R)-1-hydroxyethyl]-10-methoxy-25-oxa-16-azahexacyclo[15.7.2.01,26.02,15.05,14.07,12]hexacosa-2,4,7(12),8,10,14,20-heptaen-18,22-diyne-6,13-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
485.4 |
Polar area |
149 |
||
Complexity |
1170 |
xlogp Value |
2.3 |
|||
Heavy Count |
36 |
Rot Bonds |
2 |
|||
Hbond acc |
9 |
Hbond Donor |
5 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Effective Concentration (EC50) | 0.45 | nM |
SF-295 cells
|
Glioblastoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 0.74 | nM |
SK-MEL-5 cells
|
Cutaneous melanoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 1.1 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 4.2 | nM |
NCI-H226 cells
|
Pleural epithelioid mesothelioma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 400 | nM |
LT12 tumor cells
|
Rat leukemia
|
[2] |
Each Antibody-drug Conjugate Related to This Payload
References
