Payload Information
General Information of This Payload
| Payload ID | PAY0CJNCU |
|||||
|---|---|---|---|---|---|---|
| Name | SHR9265 |
|||||
| Synonyms |
SHR9265
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 1 (TOP1) | |||||
| Structure |
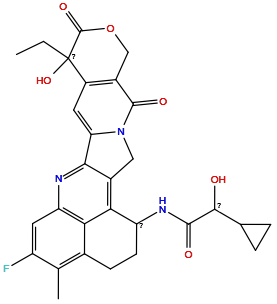
|
|||||
| Formula | C29H28FN3O6 |
|||||
| Isosmiles | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2c-1nc1cc(F)c(C)c3c1c2C(NC(=O)C(O)C1CC1)CC3 |
|||||
| InChI |
InChI=1S/C29H28FN3O6/c1-3-29(38)17-8-21-24-15(10-33(21)27(36)16(17)11-39-28(29)37)23-19(32-26(35)25(34)13-4-5-13)7-6-14-12(2)18(30)9-20(31-24)22(14)23/h8-9,13,19,25,34,38H,3-7,10-11H2,1-2H3,(H,32,35)
|
|||||
| InChIKey |
ODYAHADGPIHDQN-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
533.556 |
Polar area |
130.75 |
||
Complexity |
533.1962138 |
xlogp Value |
2.40152 |
|||
Heavy Count |
39 |
Rot Bonds |
4 |
|||
Hbond acc |
8 |
Hbond Donor |
3 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Trastuzumab rezetecan [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
61.60%
81.50% (HER2-positive BC) 55.80% (HER2-low BC) |
|||
| Patients Enrolled |
Pts were eligible if they had HER2 positive breast cancer (BC), HER2 positive gastric/GEJ carcinoma, HER2 low-expressing BC, HER2-expressing/mutated NSCLC, or other HER2-expressing/mutated solid tumors, and were refractory or intolerant to standard therapy.
|
||||
| Administration Dosage |
SHR-A1811 at doses of 1.00-8.00 mg/kg was given Q3W (IV).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04446260 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 multi-country, multi-center, open-label study to evaluate the safety, tolerability, pharmacokinetics and efficacy of SHR-A1811 in HER2 expressing or mutated advanced malignant solid tumor subjects.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05424835 | Phase Status | Phase 3 | ||
| Clinical Description |
A phase 3, multicenter, randomized, open-label, parallel controlled study of SHR-A1811 versus pyrotinib in combination with capecitabine for HER2-positive, unresectable and/or metastatic breast cancer subjects previously treated with trastuzumab and taxane.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05769010 | Phase Status | Phase 2 | ||
| Clinical Description |
A prospective, open-label explorative study of SHR-A1811 in HER2-expression advanced breast cancer with brain metastases.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05749588 | Phase Status | Phase 2 | ||
| Clinical Description |
Precision platform study of refractory triple-negative breast cancer based on molecular subtyping (a phase 2, open-label, single-center platform study).
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05671822 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 1b/2 study of SHR-A1811 combinations in patients with advanced/metastatic HER2+ gastric /gastroesophageal junction adenocarcinoma.
|
||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05635487 | Phase Status | Phase 2 | ||
| Clinical Description |
A single-arm, phase 2 study of SHR-A1811 combined with pyrotinib maleate as neoadjuvant treatment in HER2-positive breast cancer patients.
|
||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05594095 | Phase Status | Phase 2 | ||
| Clinical Description |
Precision platform study of HR+/ HER2-advanced breast cancer based on snf typing (a prospective, open-label, multi-center, phase 2 platform study).
|
||||
| Experiment 8 Reporting the Activity Date of This ADC | [8] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05353361 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 1b/2 multicenter, open-label clinical trial of SHR-A1811 injection in combination with pyrotinib or pertuzumab or SHR-1316 or paclitaxel for injection (albumin bound) in HER2-positive breast cancer.
|
||||
| Experiment 9 Reporting the Activity Date of This ADC | [9] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05349409 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 1b/2 clinical study on the dosage exploration and efficiency expansion of SHR-A1811 for injection in combination with fluzoparib capsule in HER2-expressing advanced solid tumors of patients.
|
||||
| Experiment 10 Reporting the Activity Date of This ADC | [10] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05582499 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Fudan university shanghai cancer center breast cancer precision platform series study- neoadjuvant therapy (FASCINATE-N).
|
||||
| Experiment 11 Reporting the Activity Date of This ADC | [11] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05482568 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1B/2 clinical study of the safety, tolerability, pharmacokinetics, and efficacy of injectable SHR-A1811 in combination with pyrotinib or SHR-1316 in subjects with advanced non-small cell lung cancer with HER2.
|
||||
| Experiment 12 Reporting the Activity Date of This ADC | [12] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04818333 | Phase Status | Phase 1/2 | ||
| Clinical Description |
Phase 1/2 clinical study of the safety, tolerability, pharmacokinetics, and efficacy of SHR-A1811 for injection in subjects with advanced non-small cell lung cancer who have HER2 expression, amplification, or mutation.
|
||||
| Experiment 13 Reporting the Activity Date of This ADC | [13] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04513223 | Phase Status | Phase 1 | ||
| Clinical Description |
Safety, tolerability, pharmacokinetics, and antitumour activity of SHR-A1811, in patients with HER2-expressing advanced gastric or gastroesophageal junction adenocarcinoma and colorectal cancer: a phase 1 study.
|
||||
SHR-A1904 [Phase 3]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [14] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05277168 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, single-arm, multi-center phase 1/2a clinical study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of SHR-A1904 in subjects with advanced solid tumors.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [15] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04928625 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, single-arm, multi-center phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of SHR-A1904 in patients with advanced pancreatic cancer.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [16] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04877717 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, single-arm, multi-center phase 1 clinical study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of SHR-A1904 in patients with advanced solid tumors.
|
||||
SHR-A1921 [Phase 2]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [17] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05765032 | Phase Status | Phase 1 | ||
| Clinical Description |
An open label, multicenter, phase 1b/2 study of SHR-A1921 in combination with other anti-cancer agents in patients with advanced solid tumors.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [18] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05594875 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, multi-center phase 1 clinical study on the safety, tolerability, pharmacokinetics, and clinical activity of SHR-A1921 for injection in subjects with advanced solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [19] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05154604 | Phase Status | Phase 1 | ||
| Clinical Description |
A phase 1 multi-center, open-label study to evaluate the safety, tolerability, pharmacokinetics and efficacy of SHR-a1921 in subjects with advanced malignant solid tumour.
|
||||
SHR-A1912 [Phase 1]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05113069 | Phase Status | Phase 1 | ||
| Clinical Description |
An open-label, single-arm, multicenter, phase 1 study to estimate the safety, tolerability, pharmacokinetics, and efficacy of SHR-A1912 in patients with b-cell lymphoma.
|
||||
References
