Payload Information
General Information of This Payload
| Payload ID | PAY0BRKLC |
|||||
|---|---|---|---|---|---|---|
| Name | seco-CBI-dimer |
|||||
| Synonyms |
seco-CBI-dimer
Click to Show/Hide
|
|||||
| Target(s) | Human Deoxyribonucleic acid (hDNA) | |||||
| Structure |
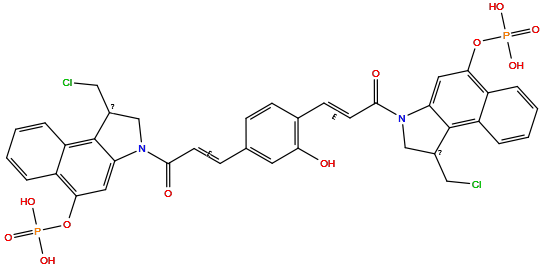
|
|||||
| Formula | C38H32Cl2N2O11P2 |
|||||
| Isosmiles | O=C(/C=C/c1ccc(/C=C/C(=O)N2CC(CCl)c3c2cc(OP(=O)(O)O)c2ccccc32)c(O)c1)N1CC(CCl)c2c1cc(OP(=O)(O)O)c1ccccc21 |
|||||
| InChI |
InChI=1S/C38H32Cl2N2O11P2/c39-18-24-20-41(30-16-33(52-54(46,47)48)26-5-1-3-7-28(26)37(24)30)35(44)13-10-22-9-11-23(32(43)15-22)12-14-36(45)42-21-25(19-40)38-29-8-4-2-6-27(29)34(17-31(38)42)53-55(49,50)51/h1-17,24-25,43H,18-21H2,(H2,46,47,48)(H2,49,50,51)/b13-10+,14-12+
|
|||||
| InChIKey |
AUOVWTYPRKEPAY-ADWQEOMMSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
825.531 |
Polar area |
194.37 |
||
Complexity |
55 |
xlogp Value |
7.4064 |
|||
Heavy Count |
55 |
Rot Bonds |
10 |
|||
Hbond acc |
7 |
Hbond Donor |
5 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Alpha-CD22-LC-K149C-10 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 68.00% (Day 12) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
In vivo efficacy of TDCs aCD22-LC-K149C-10 and aLy6E-LC-K149C-10 in the WSU-DLCL2 xenograft model. single administrations of aCD22-LC-K149C-10 (0.3 mg/kg) to mice bearing CD22-expressing WSU-DLCL2 tumor xenografts model.
|
||||
| In Vivo Model | WSU-DLCL2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 87.00 (Day 12) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
In vivo efficacy of TDCs aCD22-LC-K149C-10 and aLy6E-LC-K149C-10 in the WSU-DLCL2 xenograft model. single administrations of aCD22-LC-K149C-10 (1 mg/kg) to mice bearing CD22-expressing WSU-DLCL2 tumor xenografts model.
|
||||
| In Vivo Model | WSU-DLCL2 CDX model | ||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Anti-CD22- (LC:K149C)-SN36248 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 95.70% (Day 10) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of thio-hu anti-CD22-(LC:K149C)-SN36248 against cancer cell growth was compared with a Pinatuzumab vedotin (pina) and polatuzumab vedotin (pola) against various human cancer cell lines in vitro.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.00% (Day 15) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of thio-hu anti-CD22-(LC:K149C)-SN36248 against cancer cell growth was compared with a Pinatuzumab vedotin (pina) and polatuzumab vedotin (pola) against various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 96.70% (Day 14) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of thio-hu anti-CD22-(LC:K149C)-SN36248 against cancer cell growth was compared with a Pinatuzumab vedotin (pina) and polatuzumab vedotin (pola) against various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB.Luc-22R1.2 cells | CVCL_5711 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.20% (Day 15) | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
The inhibitory activity of thio-hu anti-CD22-(LC:K149C)-SN36248 against cancer cell growth was compared with a Pinatuzumab vedotin (pina) and polatuzumab vedotin (pola) against various human cancer cell lines in vitro.
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB.Luc cells | CVCL_5711 | ||
Alpha-CD22-LC-V205C-10 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.01 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
92.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-CD22-HC-A140C-11 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.03 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.14 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-CD22-HC-A140C-10 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.07 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.15 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-CD22-LC-K149C-11 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.08 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.25 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-Ly6E-LC-K149C-11 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
61.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
Alpha-NaPi2b-LC-K149C-11 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
9.60 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
51.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
Alpha-Ly6E-LC-K149C-10 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
52.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-NaPi2b-LC-K149C-10 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
81.00 nM
|
Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Alpha-gD-LC-K149C-10 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Burkitt lymphoma | BJAB cells | CVCL_5711 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 130 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 133 nM | Positive CD22 expression (CD22+++/++) | ||
| Method Description |
Seco-CBI-Dimer TDCs Exhibit Potent Antigen-Dependent Antiproliferation Effects in Vitro. All cell-based assay results are reported as the arithmetic mean of at least three separate runs (n = 3).
|
||||
| In Vitro Model | Diffuse large B-cell lymphoma | WSU-DLCL2 cells | CVCL_1902 | ||
References
