Payload Information
General Information of This Payload
| Payload ID | PAY0BBRSF |
|||||
|---|---|---|---|---|---|---|
| Name | KL610023 |
|||||
| Synonyms |
KL610023
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 1 (TOP1) | |||||
| Structure |
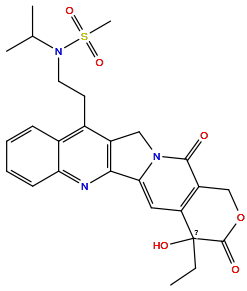
|
|||||
| Formula | C26H29N3O6S |
|||||
| Isosmiles | CCC1(O)C(=O)OCc2c1cc1n(c2=O)Cc2c-1nc1ccccc1c2CCN(C(C)C)S(C)(=O)=O |
|||||
| InChI |
InChI=1S/C26H29N3O6S/c1-5-26(32)20-12-22-23-18(13-28(22)24(30)19(20)14-35-25(26)31)16(17-8-6-7-9-21(17)27-23)10-11-29(15(2)3)36(4,33)34/h6-9,12,15,32H,5,10-11,13-14H2,1-4H3
|
|||||
| InChIKey |
PGWIUEMZGPWFBB-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
511.6 |
Polar area |
118.8 |
||
Complexity |
511.1777066 |
xlogp Value |
2.2921 |
|||
Heavy Count |
36 |
Rot Bonds |
6 |
|||
Hbond acc |
8 |
Hbond Donor |
1 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 1.36 | nM |
Calu-3 cells
|
Lung adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.505 | nM |
HCC1806 cells
|
Breast squamous cell carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.007 | nM |
NCI-N87 cells
|
Gastric tubular adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.773 | nM |
NCI-H23 cells
|
Lung adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 5.996 | nM |
BxPC-3 cells
|
Pancreatic ductal adenocarcinoma
|
[1] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Sacituzumab tirumotecan [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05347134 | Phase Status | Phase 3 | ||
| Clinical Description |
A randomized, controlled, open-label, multi-center phase 3 clinical trial of SKB264 for injection versus investigator selected regimens in patients with unresectable locally advanced, recurrent or metastatic triple-negative breast cancer who have failed second-line or above prior standard of care.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05642780 | Phase Status | Phase 2 | ||
| Clinical Description |
Amulticenter, open-label, phase 2, basket study to evaluate the efficacy and safety of SKB264 in combination with pembrolizumab in subjects with selected solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05631262 | Phase Status | Phase 2 | ||
| Clinical Description |
A multicenter, open-label, phase 2 study to evaluate the efficacy and safety of SKB264 monotherapy in selected subjects with advanced solid tumors.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05445908 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 2 clinical study of SKB264 with/without KL-A167 in patients with unresectable locally advanced, recurrent or metastatic triple-negative breast cancer (TNBC) who have not received prior systemic therapy.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [6] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05351788 | Phase Status | Phase 2 | ||
| Clinical Description |
A phase 2 clinical study of combination therapy of SKB264 in patients with advanced or metastatic non-small cell lung cancer.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 30.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 30.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.40% (Day 21) | Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.90% (Day 21) | Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
44.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 0.5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 50.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 21) | High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.60% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1.5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
51.20% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 3 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
75.60% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 1 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
78.40% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 0.3 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
98.50% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 3 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 10 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 1 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.28 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations:Calu-3 (8,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.24 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCI-N87 (5,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.41 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCI-H23 (TROP2+, 3,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H23 cells | CVCL_1547 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.70 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: HCC1806 (3,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.03 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: BxPC-3 (2,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [7] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.83 nM
|
Negative TROP2 expression (TROP2-) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCIH23 (parental, 3,000 cells per well).After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H23 cells | CVCL_1547 | ||
References
