Payload Information
General Information of This Payload
| Payload ID | PAY0AMOAM |
|||||
|---|---|---|---|---|---|---|
| Name | Nemorubicin |
|||||
| Synonyms |
Nemorubicin; 108852-90-0; Nemorubicin [INN]; Methoxymorpholino-doxorubicin; Methoxymorpholinyl doxorubicin; FCE-23762; pnu 152243; UNII-7618O47BQM; CHEBI:42053; 7618O47BQM; PNU152243A; PNU-152243; 3'-DESAMINO-3'-(2-METHOXY-4-MORPHOLINYL)-DOXORUBICIN; (1S,3S)-3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl 2,3,6-trideoxy-3-((S)-2-methoxymorpholino)-alpha-L-lyxo-hexopyranoside; 3'-deamino-3'-(2-methoxymorpholin-4-yl)doxorubicin; methoxymorpholinoDX; (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 2,3,6-trideoxy-3-[(2S)-2-methoxymorpholin-4-yl]-alpha-L-lyxo-hexopyranoside; Methoxymorpholinyldoxorubicin; DTXSID6057619; methoxymorpholinyl-doxorubicin; Nemorubicinol; methoxy-morpholinyl-doxorubicin; Fce 23762; D0K2CR; SCHEMBL24646; NEMORUBICIN [WHO-DD]; 3'-deamino-3'-(2(S)-methoxy-4-morpholinyl)doxorubicin; CHEMBL1232279; C32H37NO13; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyacetyl)-1-methoxy-10-[[2,3,6-trideoxy-3-[(2S)-2-methoxy-4-morpholinyl]-a-Llyxo- hexopyranosyl]oxy]-, (8S,10S)-; AMY10326; PNU-152243A; C32-H37-N-O13; CS-2020; PNU-156685; PNU-156686; BP-29358; HY-15794; MS-30933; Methoxymorpholinyl doxorubicin hydrochloride; A14363; F84993; A855698; Q27120450; (1S,3S)-3-GLYCOLOYL-1,2,3,4,6,11-HEXAHYDRO-3,5,12-TRIHYDROXY-10-METHOXY-6,11-DIOXO-1-NAPHTHACENYL 2,3,6-TRIDEOXY-3-((S)-2-METHOXYMORPHOLINO)-.ALPHA.-L-LYXO-HEXOPYRANOSIDE; (8S,10S)-6,8,11-Trihydroxy-10-(((2R,4S,5S,6S)-5-hydroxy-4-((S)-2-methoxymorpholino)-6-methyltetrahydro-2H-pyran-2-yl)oxy)-8-(2-hydroxyacetyl)-1-methoxy-7,8,9,10-tetrahydrotetracene-5,12-dione; 5,12-NAPHTHACENEDIONE, 7,8,9,10-TETRAHYDRO-6,8,11-TRIHYDROXY-8-(2-HYDROXYACETYL)-1-METHOXY-10-((2,3,6-TRIDEOXY-3-((2S)-2-METHOXY-4-MORPHOLINYL)-.ALPHA.-L-LYXO-HEXOPYRANOSYL)OXY)-, (8S,10S)-; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-10-((2,3,6-trideoxy-3-(2-methoxy-4-morpholinyl)-alpha-L-lyxo-hexopyranosyl)oxy)-, (8S-cis)-; 5,12-Naphthacenedione, 7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-10-[[2,3,6-trideoxy-3-[(2S)-2-methoxy-4-morpholinyl]-.alpha.-L-lyxo-hexopyranosyl]oxy]-, (8S,10S)-
Click to Show/Hide
|
|||||
| Target(s) | DNA topoisomerase 2-alpha (TOP2A) | |||||
| Structure |
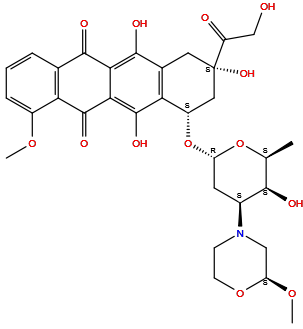
|
|||||
| Formula | C32H37NO13 |
|||||
| Isosmiles | C[C@H]1[C@H]([C@H](C[C@@H](O1)O[C@H]2C[C@@](CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N6CCO[C@@H](C6)OC)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C32H37NO13/c1-14-27(36)17(33-7-8-44-22(12-33)43-3)9-21(45-14)46-19-11-32(41,20(35)13-34)10-16-24(19)31(40)26-25(29(16)38)28(37)15-5-4-6-18(42-2)23(15)30(26)39/h4-6,14,17,19,21-22,27,34,36,38,40-41H,7-13H2,1-3H3/t14-,17-,19-,21-,22-,27+,32-/m0/s1
|
|||||
| InChIKey |
CTMCWCONSULRHO-UHQPFXKFSA-N
|
|||||
| IUPAC Name |
(7S,9S)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7-[(2R,4S,5S,6S)-5-hydroxy-4-[(2S)-2-methoxymorpholin-4-yl]-6-methyloxan-2-yl]oxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione
|
|||||
| Pharmaceutical Properties | Molecule Weight |
643.6 |
Polar area |
202 |
||
Complexity |
1160 |
xlogp Value |
1.7 |
|||
Heavy Count |
46 |
Rot Bonds |
7 |
|||
Hbond acc |
14 |
Hbond Donor |
5 |
|||
The activity data of This Payload
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Tras-Gly5-EDA-Nemo [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.80 ng/mL
|
High HER2 expression (HER2+++; 694,000 HER2 molecules/cell) | ||
| Method Description |
Briefly, cells were plated on 96-well plates in 75 uL growth medium and grown at 37°C in a humidified incubator in a 7.5% CO2 atmosphere. After one day incubation, 25 uL of 3.5-fold serial dilutions of each ADC in growth medium were added, typically resulting in final ADC concentrations from 20 ug/mL to 0.02 ng/mL.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
References
