Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0WOJVL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
BB-1701
|
|||||
| Synonyms |
BB 1701; BB-1701; BB1701
Click to Show/Hide
|
|||||
| Organization |
Baili Sikang Biomedicine (Hangzhou) Co., Ltd.
|
|||||
| Drug Status |
Phase 2
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
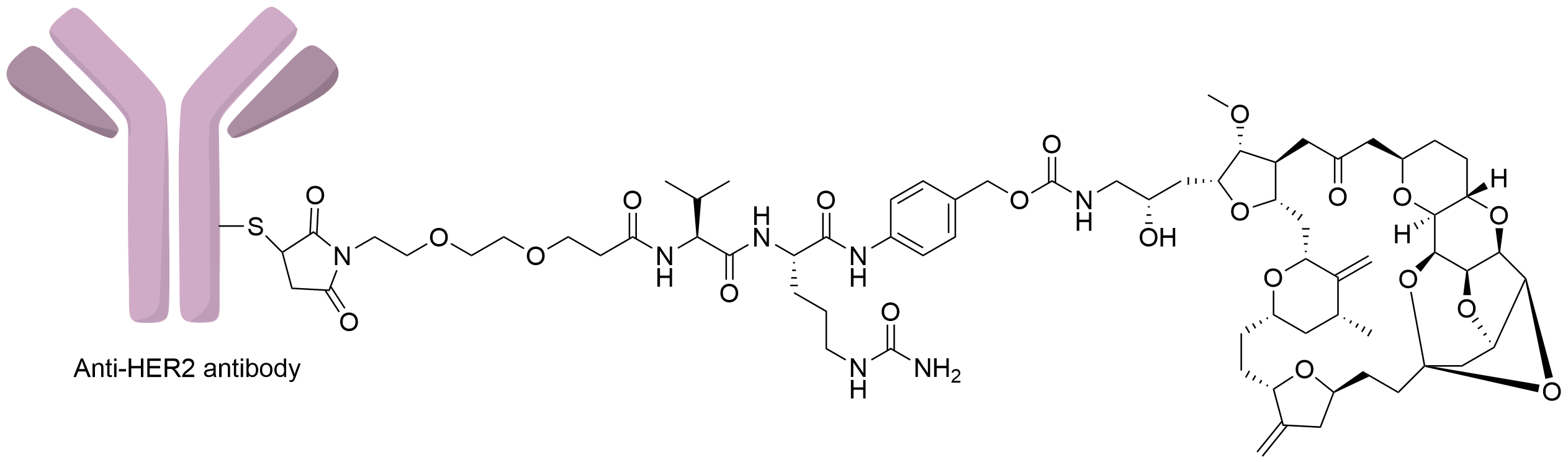
|
|||||
| Antibody Name |
Undisclosed
|
|||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2 exon 20)
|
Antigen Info | ||||
| Payload Name |
Eribulin
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Mc-PEG2-Val-Cit-PABC
|
Linker Info | ||||
| Conjugate Type |
Random Cysteines
|
|||||
| Puchem SID | ||||||
| Drugbank ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
50.00% (all)
70.60% (breast cancer) |
|||
| Patients Enrolled |
Patients with advanced/metastatic HER2-positive solid tumors, who had progressed on, or were intolerant to prior standard therapies, with ECOG PS 2, and measurable disease,.
|
||||
| Administration Dosage |
6 dose levels from 0.40 to 2.60 mg/kg Q3W.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT04257110 | Clinical Status | Phase 1 | ||
| Clinical Description | A first-in-human, open label, multiple dose, dose escalation and cohort expansion phase 1 study to investigate the safety, tolerability, pharmacokinetics and antitumor activities of bb-1701 in subjects with locally advanced/metastatic HER2 expressing solid tumors. | ||||
References
