Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SQXAL
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ARX-517
|
|||||
| Synonyms |
ARX 517; ARX-517; ARX517
Click to Show/Hide
|
|||||
| Organization |
Ambrx, Inc.
|
|||||
| Drug Status |
Phase 1
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
2
|
|||||
| Structure |
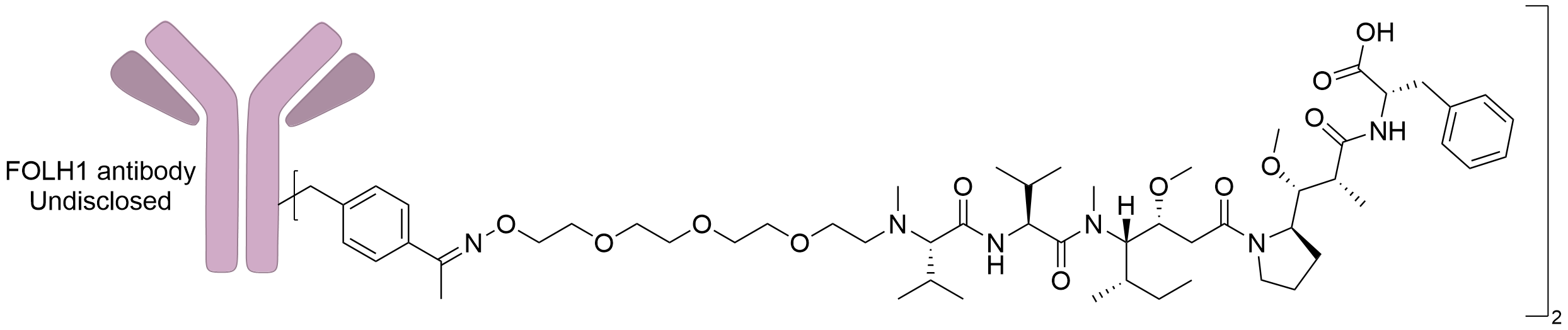
|
|||||
| Antibody Name |
Undisclosed; a humanized anti-PSMA antibody, https://aacrjournals.org/mct/article/23/12/1842/750247
|
Antibody Info | ||||
| Antigen Name |
Glutamate carboxypeptidase 2 (FOLH1)
|
Antigen Info | ||||
| Payload Name |
Monomethyl auristatin F
|
Payload Info | ||||
| Therapeutic Target |
Microtubule (MT)
|
Target Info | ||||
| Linker Name |
Oxime-PEG3
|
Linker Info | ||||
| Conjugate Type |
Non-natural Amino Acids
|
|||||
| Combination Type |
amberstatin
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04662580 | Clinical Status | Phase 1/2 | ||
| Clinical Description | A phase 1/2, multicenter, open-label, dose-escalation, and dose-expansion study to evaluate the safety, pharmacokinetics, and anti-tumor activity of ARX517, with randomized comparison to investigator's choice of treatment, in subjects with metastatic castration-resistant prostate cancer who are resistant or refractory to prior standard therapies. | ||||
References
