Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0SHGPV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
PF-06647263
|
|||||
| Synonyms |
Anti-EFNA4-ADC; Anti-EFNA4-monoclonal-antibody-calicheamicin-conjugate; PF 6647263; PF-06647263; PF06647263; PF-0667263; PF 0667263; PF0667263
Click to Show/Hide
|
|||||
| Organization |
Pfizer Inc.
|
|||||
| Drug Status |
Phase 1 (Terminated)
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4.6
|
|||||
| Structure |
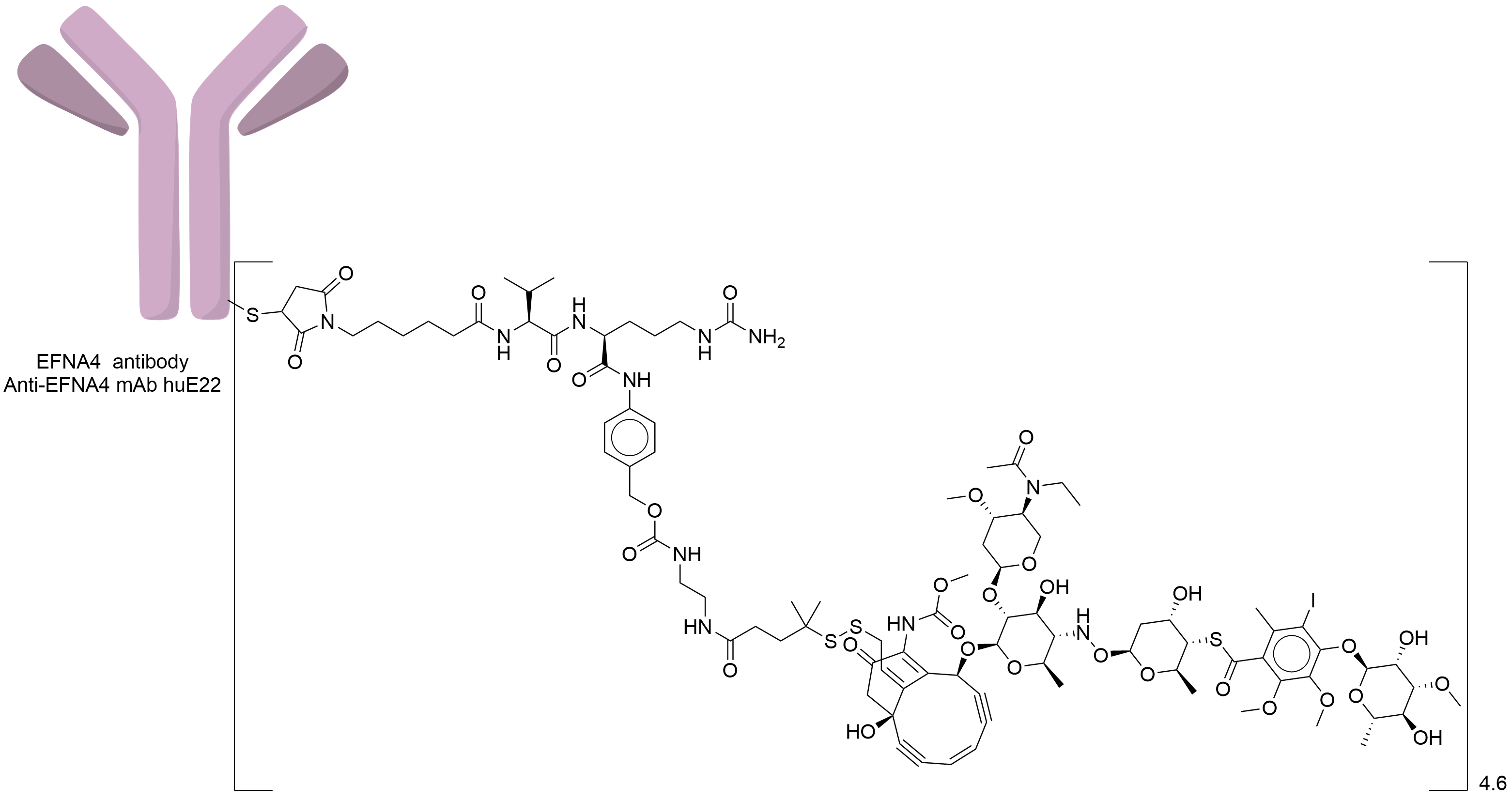
|
|||||
| Antibody Name |
Anti-EFNA4 mAb huE22
|
Antibody Info | ||||
| Antigen Name |
Ephrin-A4 (EFNA4)
|
Antigen Info | ||||
| Payload Name |
N-acetyl-gamma-calicheamicin
|
Payload Info | ||||
| Therapeutic Target |
Human Deoxyribonucleic acid (hDNA)
|
Target Info | ||||
| Linker Name |
AcButDMH
|
Linker Info | ||||
| Conjugate Type |
Random Lysines
|
|||||
| TTD ID | ||||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
10.40% (all dose-escalation groups)
9.10% (in the 0.015 mg/kg QW group ) |
|||
| Patients Enrolled |
Advanced solid tumors resistant to standard therapy or for which no standard therapy.
|
||||
| Administration Dosage |
Every 3 weeks (Q3W) or every week (QW), following a modified toxicity probability interval (mTPI) method (initial dosing: 0.015 mg/kg Q3W).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02078752 | Clinical Status | Phase 1 | ||
| Clinical Description | A first-in-human phase 1, dose escalation, safety and pharmacokinetic study of PF-06647263 in adult patients with advanced solid tumors. | ||||
| Primary Endpoint |
Six (10.00%) patients achieved a confirmed partial response and 22 (36.70%) patients had stable disease. No correlations were observed between tumor responses and EFNA4 expression levels. Study findings showed manageable safety and favorable PK for PF-06647263 administered QW at the RP2D,with preliminary evidence of limited antitumor activity in patients with TNBC and ovarian cancer.
Click to Show/Hide
|
||||
| Other Endpoint |
The RP2D was determined to be 0.015 mg/kg QW.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT02078752 | Clinical Status | Phase 1 | ||
| Clinical Description | A first-in-human phase 1, dose escalation, safety and pharmacokinetic study of PF-06647263 in adult patients with advanced solid tumors. | ||||
References
