Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0PSIFH
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
WO2022253035A1 ADC-32
|
|||||
| Synonyms |
WO2022253035A1 ADC 32
Click to Show/Hide
|
|||||
| Organization |
Sichuan Kelun Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 3 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7.5
|
|||||
| Structure |
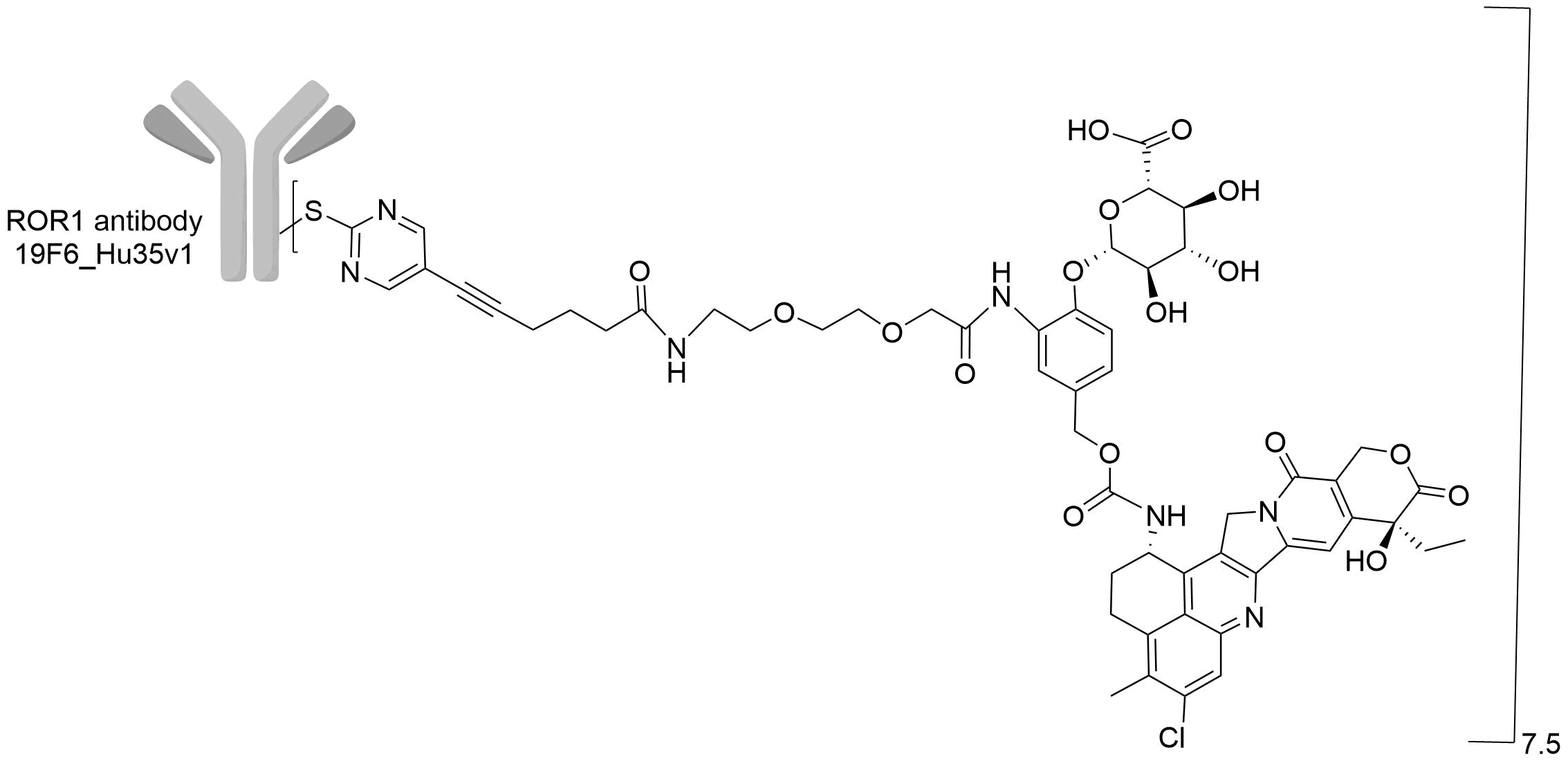
|
|||||
| Antibody Name |
19F6_Hu35v1
|
Antibody Info | ||||
| Antigen Name |
Inactive tyrosine-protein kinase transmembrane receptor ROR1 (ROR1)
|
Antigen Info | ||||
| Payload Name |
Undisclosed
|
|||||
| Linker Name |
WO2022253035A1 ADC-32 linker
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 13.50 ug/mL | Positive ROR1 expression (ROR1 +++/++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1975 cells | CVCL_1511 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 15.10 ug/mL | Positive ROR1 expression (ROR1 +++/++) | ||
| Method Description |
Cells were plated at about 500 cells per well in a 96-well plate in 100 uL of media. In vitro activity and targeted delivery of ADCs, the isotype-matched negative controls ADCs, and naked antibodies control were assessed in cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
References
