Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GRMKZ
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
huAb13v1-CZ
|
|||||
| Synonyms |
huAb13v1 CZ; huAb13v1CZ
Click to Show/Hide
|
|||||
| Organization |
AbbVie, Inc.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
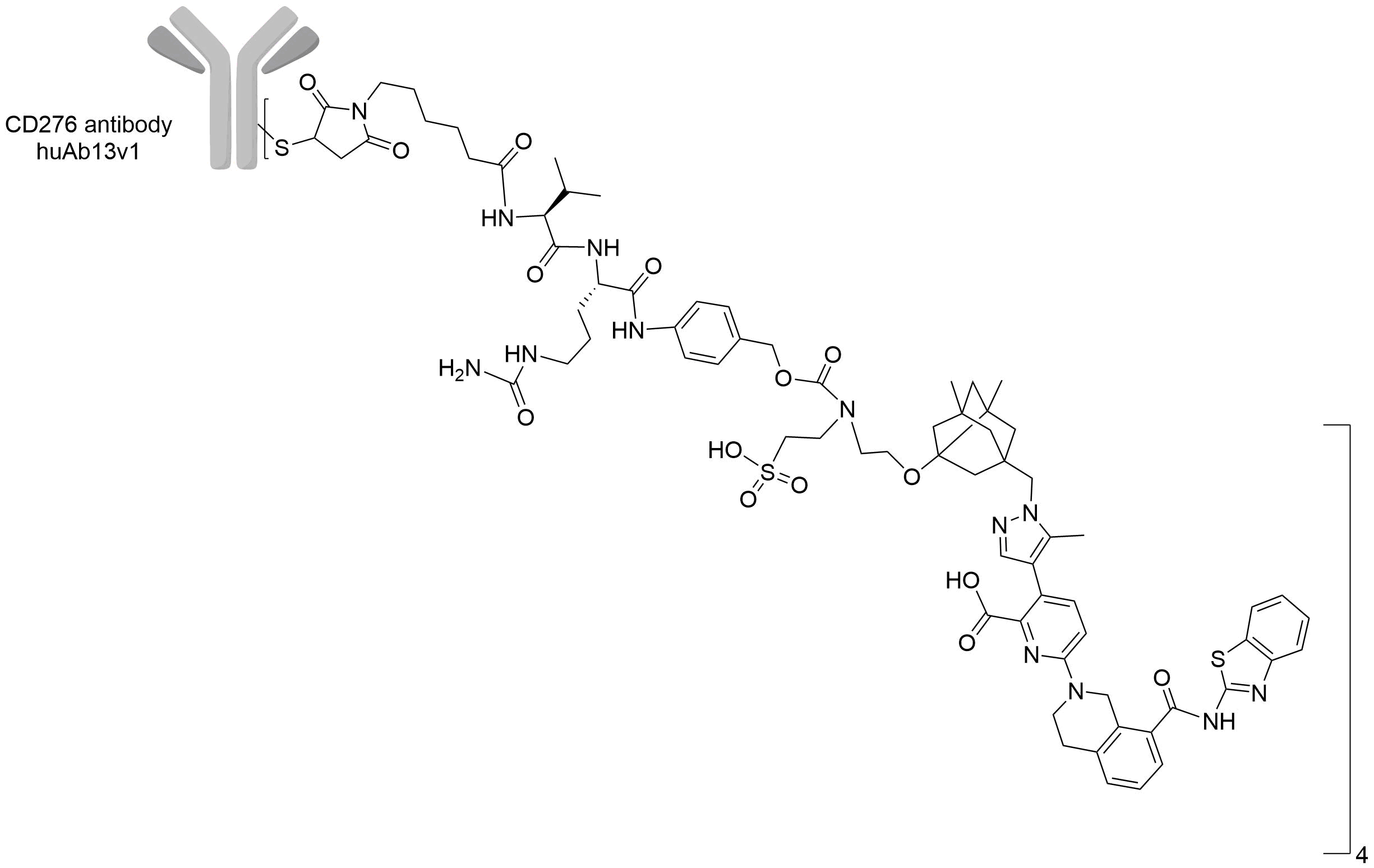
|
|||||
| Antibody Name |
huAb13v1
|
Antibody Info | ||||
| Antigen Name |
CD276 antigen (CD276)
|
Antigen Info | ||||
| Payload Name |
Undisclosed
|
|||||
| Linker Name |
huAb13v1-CZ linker
|
|||||
| Combination Type |
AbbVie's CZ
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Revealed Based on the Cell Line Data
| Standard Type | Value | Units | Cell Line | Disease Model |
|---|---|---|---|---|
| Half Maximal Effective Concentration (EC50) |
0.18
|
nM
|
NCI-H1650 cells
|
Lung adenocarcinoma
|
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 80.00% | Positive CD276 expression (CD276 +++/++) | ||
| Method Description |
The anti-tumor activity of huAb13vl as CZ or TX conjugates as purified DAR2 (E2)conjugates were characterized in enograft models of non-small cell lung cancer of human origin. The dose was ADC 10 mg/kg (QDx1/IP) plus Docetaxel 7.5 mg/kg (QDx1/IV).
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | 99.00% | Positive CD276 expression (CD276 +++/++) | ||
| Method Description |
The anti-tumor activity of huAb13vl as CZ or TX conjugates as purified DAR2 (E2)conjugates were characterized in enograft models of non-small cell lung cancer of human origin. The dose was ADC 10 mg/kg (QDx1/IP) plus Docetaxel 7.5 mg/kg (QDx1/IV).
|
||||
| In Vivo Model | NCI-H1650 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | 0.18 nM | Positive CD276 expression (CD276 +++/++) | ||
| Method Description |
The anti-tumor activity of these ADCs was tested in cytotoxicity assaysusing the NCI-H1650 non-small cell lung cancer cell line. IC50 values were determined by quantitating viable cells using a CellTiter-Glo luminescent assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H1650 cells | CVCL_1483 | ||
