Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0GNCEI
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
ADC-II-1
|
|||||
| Synonyms |
WO2022068878A1
Click to Show/Hide
|
|||||
| Organization |
Duality Biologics
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7.42
|
|||||
| Structure |
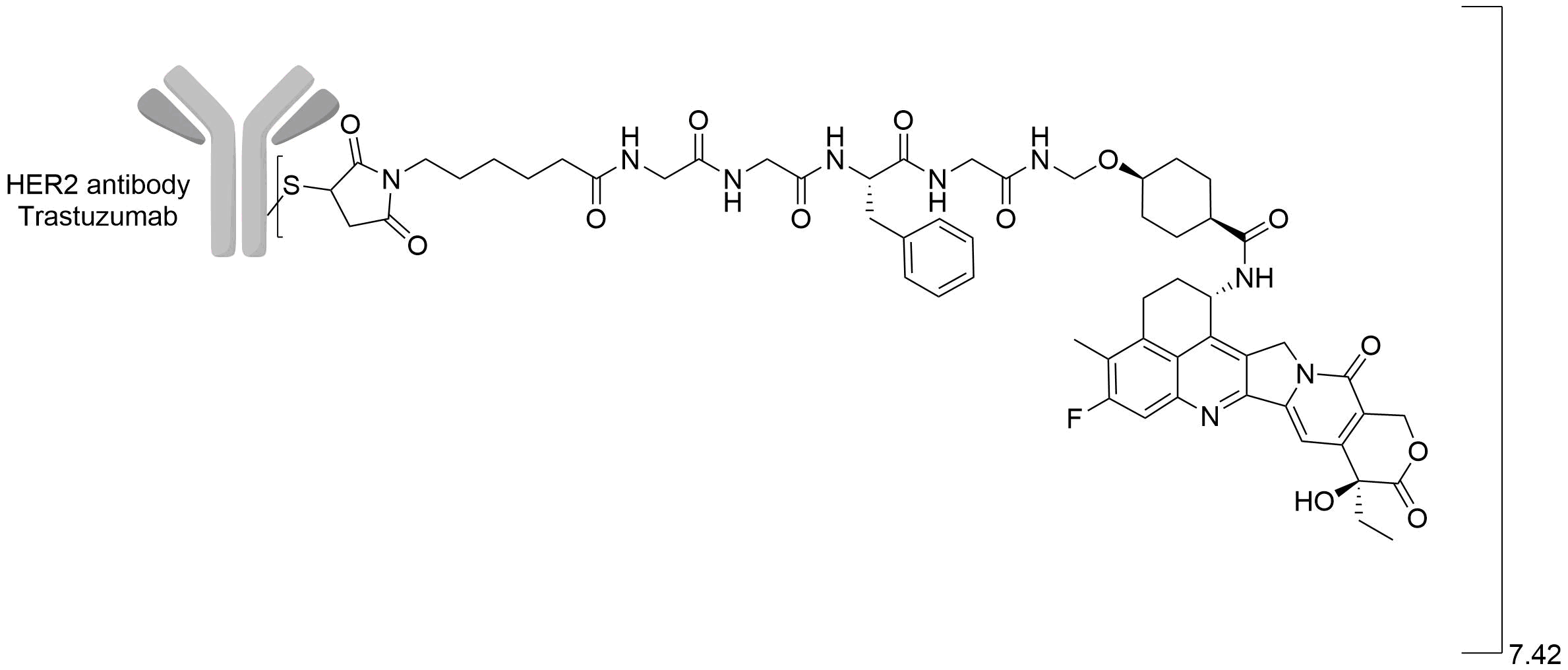
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Undisclosed
|
|||||
| Linker Name |
ADC-II-1 linker
|
|||||
General Information of The Activity Data Related to This ADC
Full List of Activity Data of This Antibody-drug Conjugate
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | 0.14 nM | High HER2 expression (HER2+++/++) | ||
| Method Description |
Cell-based in vitro assays are used to measure viability (proliferation), cytotoxicity,and induction of apoptosis of the ADC of the invention. Culturing the cells for a period from about 6 hours to about 5 days and measuring cell viability.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 500 nM | Low HER2 expression (HER2-) | ||
| Method Description |
Cell-based in vitro assays are used to measure viability (proliferation), cytotoxicity,and induction of apoptosis of the ADC of the invention. Culturing the cells for a period from about 6 hours to about 5 days and measuring cell viability.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
