Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0FDYIA
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
WO2020063676A1 ADC-21
|
|||||
| Synonyms |
WO2020063676A1_ADC 21
Click to Show/Hide
|
|||||
| Organization |
Jiangsu Hengrui Pharmaceuticals Co., Ltd.
|
|||||
| Drug Status |
Investigative
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
7.23
|
|||||
| Structure |
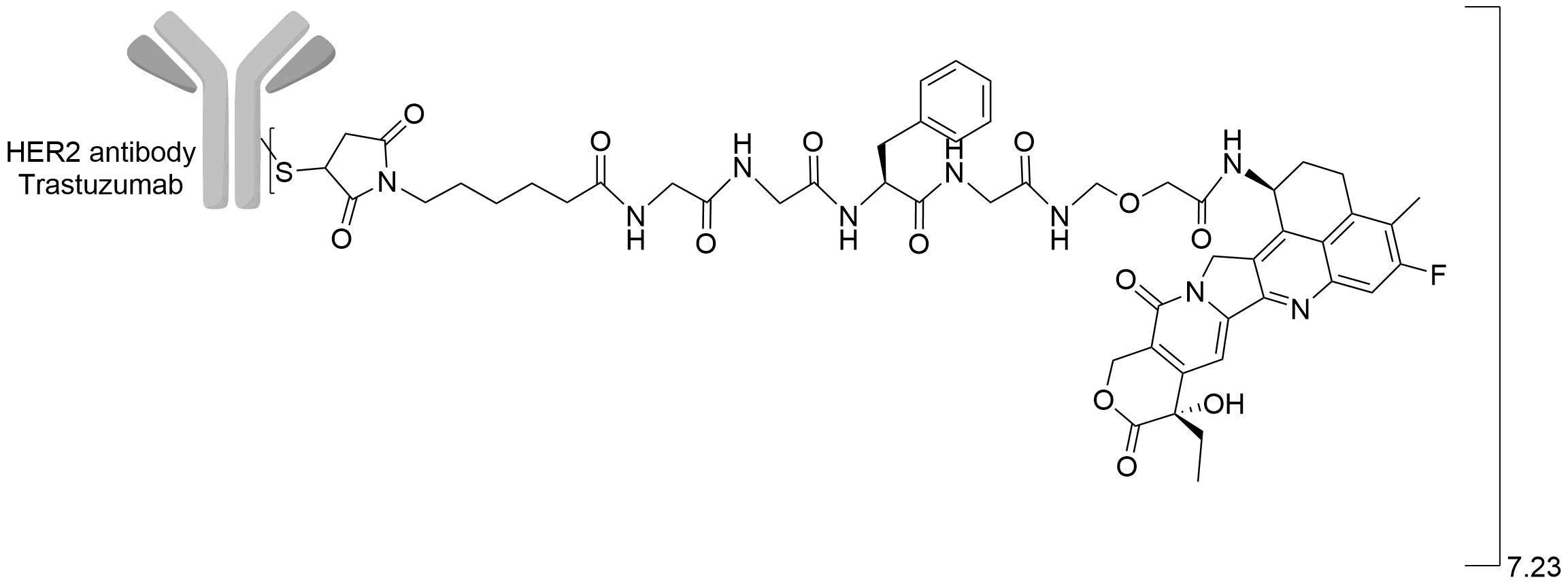
|
|||||
| Antibody Name |
Trastuzumab
|
Antibody Info | ||||
| Antigen Name |
Receptor tyrosine-protein kinase erbB-2 (HER2)
|
Antigen Info | ||||
| Payload Name |
Undisclosed
|
|||||
| Linker Name |
WO2020063676A1_ADC-21 linker
|
|||||
General Information of The Activity Data Related to This ADC
Discovered Using Cell Line-derived Xenograft Model
Full List of Activity Data of This Antibody-drug Conjugate
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 15.01% (Day 28) | Positive HER2 expression (HER2 +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 1 mg/kg, i.p.ONCE of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | SK-BR-3 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 46.22% (Day 34) | Positive HER2 expression (HER2 +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 3 mg/kg, i.p.*2 of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | JIMT-1 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 56.77% (Day 34) | Positive HER2 expression (HER2 +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 10 mg/kg, i.p.*2 of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | JIMT-1 CDX model | ||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 77.40% (Day 28) | Positive HER2 expression (HER2 +++) | ||
| Method Description |
Conjugates of the exemplary antibodies were tested using an established xenograft model implanted subcutaneous into SCID mice. Mice were randomized by body weight into treatment groups and treated once post cell inoculation with 6 mg/kg, i.p.ONCE of one of the conjugates listed above or with PBS only.
|
||||
| In Vivo Model | SK-BR-3 CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
References
