Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0DXROV
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
Cetuximab sarotalocan
|
|||||
| Brand Name |
Akalux
|
|||||
| Synonyms |
ASP-1929;RM-1929;Cetuximab-IR700;Cetuximab Sarotalocan Sodium
Click to Show/Hide
|
|||||
| Organization |
Rakuten Medical, Inc.
|
|||||
| Drug Status |
Approved (FDA): Sep, 2020
|
|||||
| Indication |
In total 2 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
1.3 to 3.8
|
|||||
| Structure |
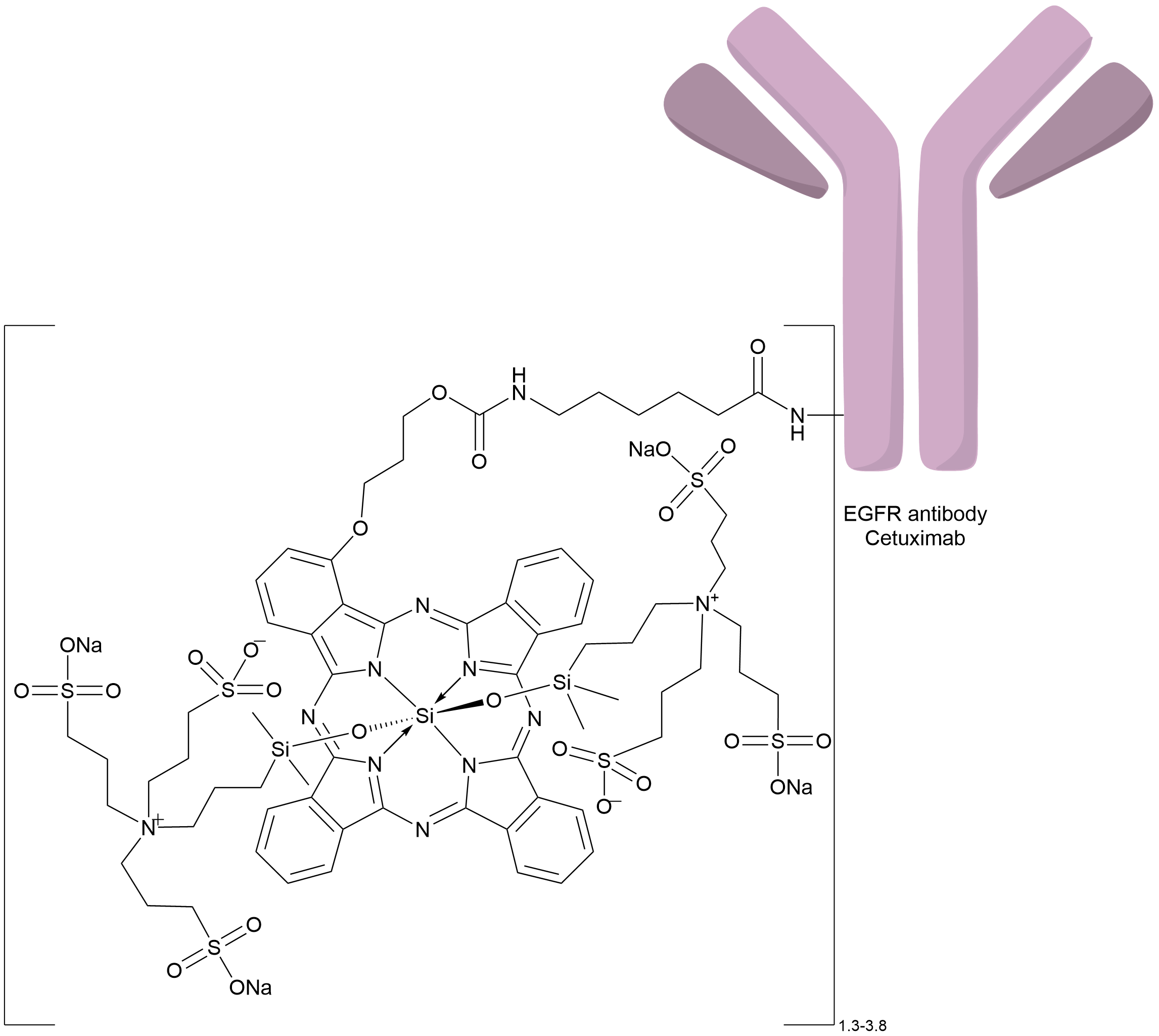
|
|||||
| Antibody Name |
Cetuximab
|
Antibody Info | ||||
| Antigen Name |
Epidermal growth factor receptor (EGFR)
|
Antigen Info | ||||
| Payload Name |
IRDye 700DX
|
Payload Info | ||||
| Linker Name |
Linear alkyl/alkoxy linker
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
| Combination Type |
Sarotalocan
|
|||||
| Puchem SID | ||||||
| ChEBI ID | ||||||
