Antibody-drug Conjugate Information
General Information of This Antibody-drug Conjugate (ADC)
| ADC ID |
DRG0BGJNX
|
|||||
|---|---|---|---|---|---|---|
| ADC Name |
TAK-500
|
|||||
| Synonyms |
TAK 500; TAK-500; TAK500
Click to Show/Hide
|
|||||
| Organization |
Takeda Pharmaceutical Co., Ltd.
|
|||||
| Drug Status |
Phase 1/2 (Terminated)
|
|||||
| Indication |
In total 1 Indication(s)
|
|||||
| Drug-to-Antibody Ratio |
4
|
|||||
| Structure |
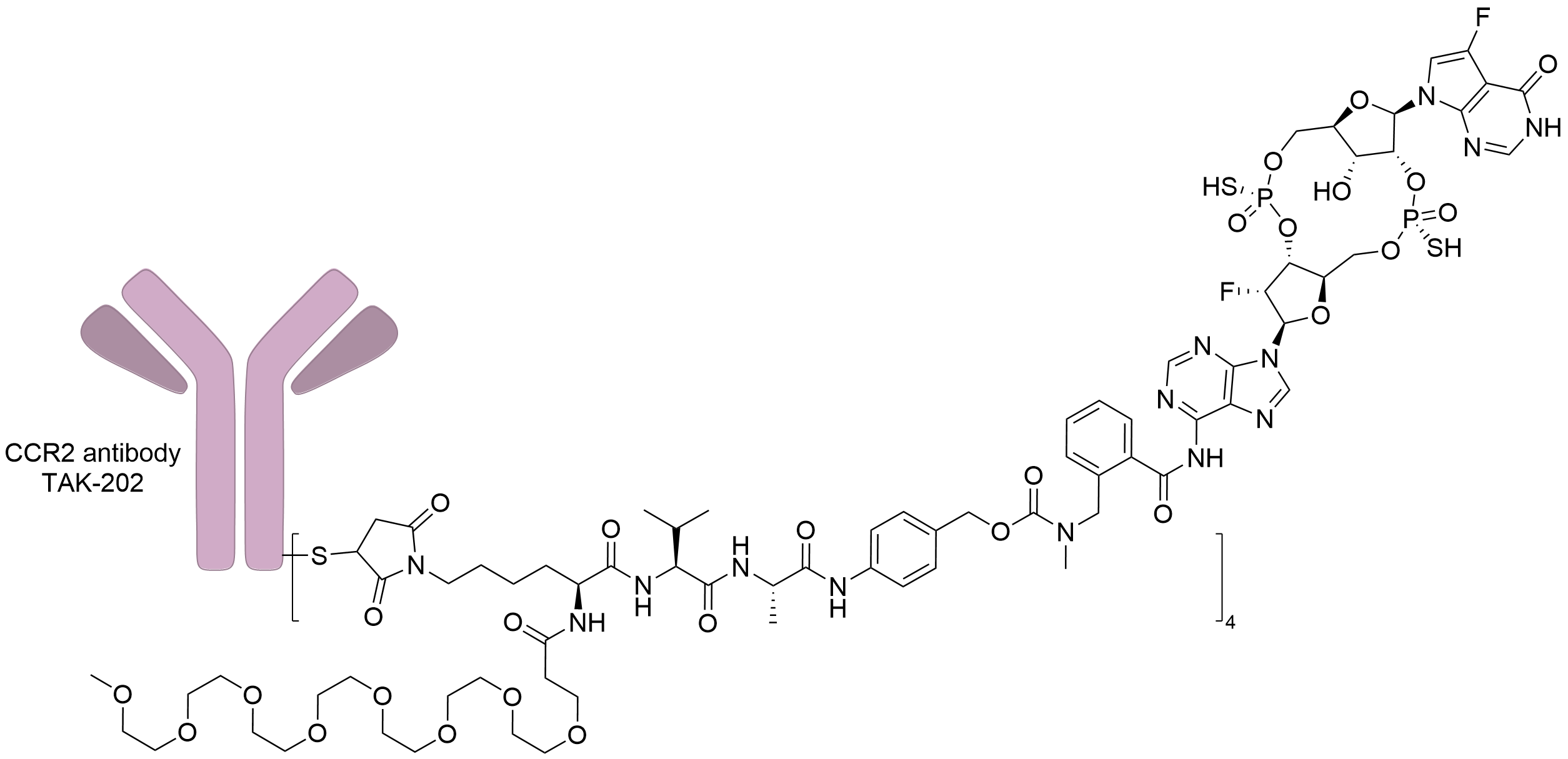
|
|||||
| Antibody Name |
TAK-202
|
Antibody Info | ||||
| Antigen Name |
C-C chemokine receptor type 2 (CCR2)
|
Antigen Info | ||||
| Payload Name |
STING agonist of TAK500
|
Payload Info | ||||
| Therapeutic Target |
Stimulator of interferon genes protein (STING1)
|
Target Info | ||||
| Linker Name |
Maleimido-caproyl
|
Linker Info | ||||
| Conjugate Type |
Undisclosed
|
|||||
General Information of The Activity Data Related to This ADC
Identified from the Human Clinical Data
Full List of Activity Data of This Antibody-drug Conjugate
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04879849 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, phase 1, dose-escalation study to evaluate the safety and preliminary antitumor activity of TAK-676 with pembrolizumab following radiation therapy in the treatment of non-small-cell lung cancer, triple-negative breast cancer, or squamous-cell carcinoma of the head and neck that has progressed on checkpoint inhibitors. | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT04420884 | Clinical Status | Phase 1 | ||
| Clinical Description | An open-label, dose escalation, phase 1 study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of TAK-676 as a single agent and in combination with pembrolizumab in adult patients with advanced or metastatic solid tumors. | ||||
References
