Payload Information
General Information of This Payload
| Payload ID | PAY0ZAWKE |
|||||
|---|---|---|---|---|---|---|
| Name | Triptolide |
|||||
| Synonyms |
Triptolide; 38748-32-2; Triptolid; PG490; NSC 163062; NSC-163062; UNII-19ALD1S53J; 19ALD1S53J; CHEBI:9747; CHEMBL463763; C20H24O6; PG-490; (1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one; (5bS,6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-Hydroxy-8a-isopropyl-10b-methyl-1,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(2H)-one; (6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-hydroxy-8a-isopropyl-10b-methyl-1,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(2H)-one.; SMR000466307; Trisoxireno(4b,5:6,7:8a,9)phenanthro(1,2-c)furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-; NSC163062; Triptolide, 1; (3BS,4AS,5AS,6R,6AR,7AS,7BS,8AS,8BS)-3B,4,4A,6,6A,7A,7B,8B,9,10-DECAHYDRO-6-HYDROXY-8B-METHYL-6A-(1-METHYLETHYL)TRISOXIRENO(4B,5:6,7:8A,9)PHENANTHRO(1,2-C)FURAN-1(3H)-ONE; (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-3b,4,4a,6,6a,7a,7b,8b,9,10-Decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)trisoxireno[4b,5:6,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one; Trisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-; MFCD00210565; CPD000466307; TRIPTOLIDE [MI]; D0I6LH; TRIPTOLIDE [WHO-DD]; BSPBio_001595; KBioGR_000315; KBioSS_000315; MLS000759410; MLS001424107; MLS006010844; SCHEMBL413634; DTXSID5041144; EX-A7744A; KBio2_000315; KBio2_002883; KBio2_005451; KBio3_000629; KBio3_000630; DFBIRQPKNDILPW-CIVMWXNOSA-N; Bio2_000315; Bio2_000795; HMS1361P17; HMS1791P17; HMS1989P17; HMS2051N13; HMS3402P17; 144539-79-7; TPL; BDBM50241049; NSC839303; s3604; AKOS022168197; AM84923; CCG-100957; CS-0286; DB12025; NC00207; NSC-839303; IDI1_034065; NCGC00163411-01; NCGC00163411-02; NCGC00163411-03; NCGC00163411-07; BP-25386; BS-16697; HY-32735; NCI60_001223; LS-157717; T2899; C09204; AB00639938-06; AB00639938-08; Q906351; Q-100450; BRD-K39484304-001-02-5; BRD-K39484304-001-06-6; BRD-K39484304-001-16-5; Triptolide, Tripterygium wilfordii - CAS 38748-32-2; (1S,2S,4S,5S,7R,8R,9S,11S)-8-Hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one; (3BS,4AS,5AS,6R,6AR,7AS,7BS,8AS,8BS)-3B,4,4A,6,6A,7A,7B,8B,9,10-DECAHYDRO-6-HYDROXY-6A-ISOPROPYL-8B-METHYLTRISOXIRENO(6,7:8A,9:4B,5)PHENANTHRO(1,2-C)FURAN-1(3H)-ONE; (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-6-hydroxy-8b-methyl-6a-(propan-2-yl)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydrotrisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one; (5bS,6aS,7aS,8R,8aR,9aS,9bS,10aS,10bS)-8-hydroxy-8a-isopropyl-10b-methyl-2,5,5b,6,6a,8,8a,9a,9b,10b-decahydrotris(oxireno)[2',3':4b,5;2'',3'':6,7;2''',3''':8a,9]phenanthro[1,2-c]furan-3(1H)-one; Trisoxireno(4b,5:6,7:8a,9)phenanthro(1,2-c)furan-1(3H)-one,3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-,(3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-; Trisoxireno[4b,7:8a,9]phenanthro[1,2-c]furan-(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, [3bR-(3b.alpha.,4a.alpha.,5aS*,6.beta.,6a.beta.,7a.beta.,7b.alpha.,8aS*,8b.beta.)]-; Trisoxireno[4b,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, [3bR-(3b.alpha.,4a.alpha.,5aS*,6.beta.,6a.beta.,7a.beta.,7b.alpha.,8aS*,8b.beta.)]-
Click to Show/Hide
|
|||||
| Target(s) | Nuclear factor NF-kappa-B p105 subunit (NFKB1) | |||||
| Structure |
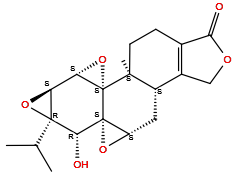
|
|||||
| Formula | C20H24O6 |
|||||
| Isosmiles | CC(C)[C@@]12[C@@H](O1)[C@H]3[C@@]4(O3)[C@]5(CCC6=C([C@@H]5C[C@H]7[C@]4([C@@H]2O)O7)COC6=O)C |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C20H24O6/c1-8(2)18-13(25-18)14-20(26-14)17(3)5-4-9-10(7-23-15(9)21)11(17)6-12-19(20,24-12)16(18)22/h8,11-14,16,22H,4-7H2,1-3H3/t11-,12-,13-,14-,16+,17-,18-,19+,20+/m0/s1
|
|||||
| InChIKey |
DFBIRQPKNDILPW-CIVMWXNOSA-N
|
|||||
| IUPAC Name |
(1S,2S,4S,5S,7R,8R,9S,11S,13S)-8-hydroxy-1-methyl-7-propan-2-yl-3,6,10,16-tetraoxaheptacyclo[11.7.0.02,4.02,9.05,7.09,11.014,18]icos-14(18)-en-17-one
|
|||||
| Pharmaceutical Properties | Molecule Weight |
360.4 |
Polar area |
84.1 |
||
Complexity |
819 |
xlogp Value |
0.2 |
|||
Heavy Count |
26 |
Rot Bonds |
1 |
|||
Hbond acc |
6 |
Hbond Donor |
1 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.0001 | ug/mL |
HT-29 cells
|
Colon adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.0013 | ug/mL |
A-549 cells
|
Lung adenocarcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
MDA-MB-468 cells
|
Breast adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
HCT 116 cells
|
Colon carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.09 | nM |
SNB-75 cells
|
Glioblastoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 10.3 | nM |
KBM5 cells
|
Chronic myelogenous leukemia
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.86 | nM |
BT-549 cells
|
Breast ductal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10.96 | nM |
CCRF-CEM cells
|
T acute lymphoblastic leukemia
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | >10000 | nM |
A549/DDP cells
|
Lung adenocarcinoma
|
[5] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 103.51 | nM |
SNB-19 cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 11.19 | nM |
Malme-3M cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 11.35 | nM |
NCI-H322M cells
|
Minimally invasive lung adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 111 | nM |
NKM-1 cells
|
Acute myeloid leukemia
|
||
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.39 | nM |
UO-31 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 12.65 | nM |
UACC-257 cells
|
Melanoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 1200 | nM |
MDCK cells
|
Normal
|
[6] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 13.55 | nM |
M14 cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 13.96 | nM |
HCT 15 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 13.96 | nM |
TK-10 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 14 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 14 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 14 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 14 | nM |
SJRH30 cells
|
Alveolar rhabdomyosarcoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 14 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[8] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.09 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.49 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 14.96 | nM |
Hs 578T cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 140 | nM |
Jurkat cells
|
T acute lymphoblastic leukemia
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 15 | nM |
SGC-7901 cells
|
Gastric carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 15 | nM |
NCI-H1650 cells
|
Lung adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 16 | nM |
L02 cells
|
Cervical carcinoma
|
[9] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 16.94 | nM |
786-O cells
|
Renal cell carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 163 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 17 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 17.02 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 17.34 | nM |
SR cells
|
Leukemia
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 17.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 18 | nM |
SMMC-7721 cells
|
Hepatocellular carcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 18.03 | nM |
A498 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 18.11 | nM |
PC-3 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 18.28 | nM |
HCC 2998 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 18.3 | nM |
PC-3 cells
|
Prostate carcinoma
|
[10] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 18.71 | nM |
T-47D cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 19 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[11] | |
| Half Maximal Inhibitory Concentration (IC50) | 19 | nM |
NCI-H1975 cells
|
Lung adenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 19 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
NCI-H1975 cells
|
Lung adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
BGC-823 cells
|
Stomach adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.1 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[11] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 2.582 | nM |
HCT 116 cells
|
Colon carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
PC-3 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
PC-3 cells
|
Prostate carcinoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
PC-3 cells
|
Prostate carcinoma
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
PC-3 cells
|
Prostate carcinoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
Bel-7402 cells
|
Hepatoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 20.14 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 20.3 | nM |
PC-3 cells
|
Prostate carcinoma
|
[15] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 20.32 | nM |
OVCAR-8 cells
|
High grade ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 20.99 | nM |
EKVX cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 200 | nM |
PANC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 200 | nM |
MKN-28 cells
|
Gastric epithelial carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 21 | nM |
PC-3 cells
|
Prostate carcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 21 | nM |
L02 cells
|
Cervical carcinoma
|
[5] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 21.38 | nM |
OVCAR-5 cells
|
Ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 21.63 | nM |
NCI-H226 cells
|
Pleural epithelioid mesothelioma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 22 | nM |
786-O cells
|
Renal cell carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 22 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 23 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 23 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[8] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 23.01 | nM |
HOP-92 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 24 | nM |
DU145 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 24 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[2] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 24.38 | nM |
DU145 cells
|
Prostate carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 24.89 | nM |
SF268 cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 26.06 | nM |
NCI-ADR-RES cells
|
High grade ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 27 | nM |
HaCaT cells
|
Normal
|
[9] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 27.35 | nM |
KM12 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 27.86 | nM |
SF-295 cells
|
Glioblastoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 28 | nM |
HO-8910 cells
|
Endocervical adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 29 | nM |
HCT 15 cells
|
Colon adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 3 | nM |
HCT 116 cells
|
Colon carcinoma
|
[8] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 3.221 | nM |
NCI-H23 cells
|
Lung adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 30 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[17] | |
| Half Maximal Inhibitory Concentration (IC50) | 30 | nM |
HBE1 cells
|
Normal
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 33 | nM |
U-251MG cells
|
Astrocytoma
|
[14] | |
| Half Maximal Inhibitory Concentration (IC50) | 33 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 37.7 | nM |
Hepatocyte cells
|
Normal
|
Undisclosed | [18] |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 4.207 | nM |
UACC-62 cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 4.266 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 4.699 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.7 | nM |
HCT 116 cells
|
Colon carcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 43 | nM |
PC-3 cells
|
Prostate carcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 43 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 43 | nM |
KB cells
|
Human papillomavirus-related endocervical adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 43.3 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[18] | |
| Half Maximal Inhibitory Concentration (IC50) | 44 | nM |
Human umbilical vein endothelial cells(HUVEC)
|
Normal
|
Undisclosed | [9] |
| Half Maximal Inhibitory Concentration (IC50) | 46 | nM |
HCT 116 cells
|
Colon carcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 468 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 47 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 47 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[9] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 47.42 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 49 | nM |
U-251MG cells
|
Astrocytoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 49 | nM |
NCI-H1299 cells (MMAE resistant)
|
Lung large cell carcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
B16-F10 cells
|
Mouse melanoma
|
[8] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 5.14 | nM |
LOX IMVI cells
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 5.272 | nM |
NCI-H460 cells
|
Lung large cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 5.861 | nM |
OVCAR-3 cells (FZD7 overexpression)
|
Ovarian serous adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 50 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 50 | nM |
NIH3T3 cells
|
Normal
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 51 | nM |
MDA-MB-231 cells (5T4 overexpression)
|
Breast adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 52 | nM |
SW1116 cells
|
Colon adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 56 | nM |
PANC-1 cells
|
Pancreatic ductal adenocarcinoma
|
[5] | |
| Half Maximal Inhibitory Concentration (IC50) | 59 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 6 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[12] | |
| Half Maximal Inhibitory Concentration (IC50) | 6 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[13] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.026 | nM |
Caki-1 cells
|
Clear cell renal cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.368 | nM |
SK-MEL-28 cells (BRAF inhibitor resistant)
|
Cutaneous melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.427 | nM |
SK-MEL-2 cells (MEK inhibitor-resistant)
|
Melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.668 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.792 | nM |
ACHN cells
|
Renal adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.934 | nM |
MDA-MB-435 cells
|
Amelanotic melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 6.966 | nM |
SK-MEL-5 cells
|
Cutaneous melanoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 60.95 | nM |
U-251MG cells
|
Astrocytoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.015 | nM |
HOP-62 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.129 | nM |
IGROV-1 cells
|
Ovarian endometrioid adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 7.2 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[10] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.447 | nM |
SF539 cells
|
Gliosarcoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.586 | nM |
MDA-N cells
|
Breast carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.638 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.762 | nM |
COLO 205 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.87 | nM |
RXF 393 cells
|
Renal carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 7.925 | nM |
OVCAR-4 cells
|
Ovarian adenocarcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 8.299 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 8.3 | nM |
KBM5 cells
|
Chronic myelogenous leukemia
|
[4] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 8.61 | nM |
SN12C cells
|
Renal cell carcinoma
|
[3] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 8.81 | nM |
NCI-H522 cells
|
Non-small cell lung carcinoma
|
[3] | |
| Half Maximal Inhibitory Concentration (IC50) | 87 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 9 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 9.7 | nM |
LNCaP cells
|
Prostate carcinoma
|
[15] | |
| Half Maximal Inhibitory Concentration (IC50) | 9200 | nM |
Embryonic fibroblast MEF cells
|
Normal
|
Undisclosed | [4] |
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Cet-TPL [Investigative]
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 72.17% (Day 13) | Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
When subcutaneous xenograft tumors reached 1.5 cm3, they were serially passaged in NSG mice by subcutaneous transplant (0.10-0.12 g, 2x2 mm) under general anesthesia. Mouse treatment was performed by intraperitoneal injection of vehicle (PBS), 50 mg/kg Cet-TPL in <300 uL PBS twice/week for about 2-3 weeks.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: PDX1) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 91.89% (Day 15) | Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
When subcutaneous xenograft tumors reached 1.5 cm3, they were serially passaged in NSG mice by subcutaneous transplant (0.10-0.12 g, 2x2 mm) under general anesthesia. Mouse treatment was performed by intraperitoneal injection of vehicle (PBS), 50 mg/kg Cet-TPL in <300 uL PBS twice/week for about 2-3 weeks.
|
||||
| In Vivo Model | Lung adenocarcinoma PDX model (PDX: PDX1) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 12.39% (Day 15) | Negative EGFR expression (EGFR -) | ||
| Method Description |
When subcutaneous xenograft tumors reached 1.5 cm3, they were serially passaged in NSG mice by subcutaneous transplant (0.10-0.12 g, 2x2 mm) under general anesthesia. Mouse treatment was performed by intraperitoneal injection of vehicle (PBS), 50 mg/kg Cet-TPL in <300 uL PBS twice/week for about 2-3 weeks.
|
||||
| In Vivo Model | H520 CDX model | ||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H520 cells | CVCL_1566 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 64.00% (Day 18) | Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
When subcutaneous xenograft tumors reached 1.5 cm3, they were serially passaged in NSG mice by subcutaneous transplant (0.10-0.12 g, 2x2 mm) under general anesthesia. Mouse treatment was performed by intraperitoneal injection of vehicle (PBS), 50 mg/kg Cet-TPL in <300 uL PBS twice/week for about 2-3 weeks.
|
||||
| In Vivo Model | A549 CDX model | ||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.80% (Day 24) | Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
When subcutaneous xenograft tumors reached 1.5 cm3, they were serially passaged in NSG mice by subcutaneous transplant (0.10-0.12 g, 2x2 mm) under general anesthesia. Mouse treatment was performed by intraperitoneal injection of vehicle (PBS), 50 mg/kg Cet-TPL in <300 uL PBS twice/week for about 2-3 weeks.
|
||||
| In Vivo Model | SCC6 CDX model | ||||
| In Vitro Model | Squamous cell carcinoma | UM-SCC-6 cells | CVCL_7773 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
6.25 ug/mL - 12.5 ug/mL
|
Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
For proliferation assay, cells were seeded in 96-well plates in four to six replicates at densities of 2000 cells per well; after 24 h, 3.125-100 ug/mL IgG, Cet, and Cet-TPL were added to wells, respectively, and further incubated with cells for 72 h.
|
||||
| In Vitro Model | Squamous cell carcinoma | UM-SCC-6 cells | CVCL_7773 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.50 ug/mL
|
Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
For proliferation assay, cells were seeded in 96-well plates in four to six replicates at densities of 2000 cells per well; after 24 h, 3.125-100 ug/mL IgG, Cet, and Cet-TPL were added to wells, respectively, and further incubated with cells for 72 h.
|
||||
| In Vitro Model | Lung adenocarcinoma | A-549 cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
12.50 ug/mL
|
Positive EGFR expression (EGFR +++/++) | ||
| Method Description |
For proliferation assay, cells were seeded in 96-well plates in four to six replicates at densities of 2000 cells per well; after 24 h, 3.125-100 ug/mL IgG, Cet, and Cet-TPL were added to wells, respectively, and further incubated with cells for 72 h.
|
||||
| In Vitro Model | Lung large cell carcinoma | NCI-H1299 cells | CVCL_0060 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [20] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 100.00 ug/mL | Negative EGFR expression (EGFR -) | ||
| Method Description |
For proliferation assay, cells were seeded in 96-well plates in four to six replicates at densities of 2000 cells per well; after 24 h, 3.125-100 ug/mL IgG, Cet, and Cet-TPL were added to wells, respectively, and further incubated with cells for 72 h.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | NCI-H520 cells | CVCL_1566 | ||
Y-TR1 SMCC [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
15.00 ug/mL
|
Positive CD26 expression (CD26+++/++) | ||
| Method Description |
Viability assay of Jurkat cells incubated for 72 h with ADC.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Y-TR1 GMBS [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.00 ug/mL
|
Positive CD26 expression (CD26+++/++) | ||
| Method Description |
Viability assay of Jurkat cells incubated for 72 h with ADC.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
Y-TR1 SPDP [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [21] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
35.00 ug/mL
|
Positive CD26 expression (CD26+++/++) | ||
| Method Description |
Viability assay of Jurkat cells incubated for 72 h with ADC.
|
||||
| In Vitro Model | T acute lymphoblastic leukemia | Jurkat cells | CVCL_0065 | ||
References
