Payload Information
General Information of This Payload
| Payload ID | PAY0YXMEM |
|||||
|---|---|---|---|---|---|---|
| Name | Fluticasone propionate |
|||||
| Synonyms |
FLUTICASONE PROPIONATE; Flovent; 80474-14-2; Cutivate; Flixotide; Flonase; Flixonase; Flovent HFA; Flusonal; Fluspiral; Flunase; Flutide; atemur; Asmatil; Axotide; Brethal; Fluinol; Flutivate; Flixotide Disk; Flixotide Disks; Flovent Diskus; Flovent Diskus 50; Flixotide Inhaler; Flovent Diskus 100; Flovent Diskus 250; Cultivate; Inalacor; Rinosone; Trialona; Ubizol; Zoflut; Flixonase Nasal Spray; Flonase Aq; Fluticasonpropionat Allen; Xhance; Flovent-hfa; CCI-18781; ArmonAir RespiClick; CCI 18781; Fluticasone (propionate); C25H31F3O5S; UNII-O2GMZ0LF5W; Fluticasone-17-propionate; O2GMZ0LF5W; cci18781; Fluticasone propionate [USAN]; ArmonAir RespiClickTM; DTXSID8045511; CHEBI:31441; Flonase Allergy Relief; NSC-759889; Fluticasone propionate (Flonase, Veramyst); PF-00241939; DTXCID801476602; Fluticasone propionate [USAN:USP]; NSC 759889; fluticasone proprionate; fluticasone 17-propionate; MFCD00866007; ADVAIR COMPONENT FLUTICASONE PROPIONATE; DYMISTA COMPONENT FLUTICASONE PROPIONATE; ADVAIR HFA COMPONENT FLUTICASONE PROPIONATE; FLUTICASONE PROPIONATE COMPONENT OF ADVAIR; FLUTICASONE PROPIONATE COMPONENT OF DYMISTA; LIPO-102 COMPONENT FLUTICASONE PROPIONATE; Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, (6alpha,11beta,16alpha,17alpha)-S-(fluoromethyl) ester; S-(Fluoromethyl) 6alpha,9-difluoro-11beta,17-dihydroxy-16alpha-methyl-3-oxoandrosta-1,4-diene-17beta-carbothioate, 17-propionate; FLUTICASONE PROPIONATE COMPONENT OF ADVAIR HFA; AIRDUO RESPICLICK COMPONENT FLUTICASONE PROPIONATE; FLUTICASONE PROPIONATE COMPONENT OF AIRDUO RESPICLICK; FLUTICASONE PROPIONATE (MART.); FLUTICASONE PROPIONATE [MART.]; FLUTICASONE PROPIONATE (USP-RS); FLUTICASONE PROPIONATE [USP-RS]; [(6S,8S,9R,10S,11S,13S,14S,16R,17R)-6,9-difluoro-17-(fluoromethylsulfanylcarbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate; FLUTICASONE PROPIONATE (EP MONOGRAPH); FLUTICASONE PROPIONATE [EP MONOGRAPH]; FLUTICASONE PROPIONATE (USP MONOGRAPH); FLUTICASONE PROPIONATE [USP MONOGRAPH]; Propionate, Fluticasone; Fluxonal; Skyron; C25-H31-F3-O5-S; Flovent Rotadisk; CCI-187881; NCGC00016943-01; Cutivate (TN); 6alpha,9-difluoro-17beta-(((fluoromethyl)sulfanyl)carbonyl)-11beta-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17alpha-yl propanoate; 6alpha,9-difluoro-17beta-{[(fluoromethyl)sulfanyl]carbonyl}-11beta-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17alpha-yl propanoate; Flonase (TN); Flovent (TN); CAS-80474-14-2; fluticasone-propionate; ARMONAIR DIGIHALER; Prestwick0_000997; Prestwick1_000997; Prestwick2_000997; Prestwick3_000997; SCHEMBL4068; CHEMBL1473; BSPBio_001093; MLS001424085; Fluticasone propionate- Bio-X; SPBio_002984; BPBio1_001203; GTPL7080; WMWTYOKRWGGJOA-CENSZEJFSA-N; Fluticasone propionate (JAN/USP); FN-25; HMS1571G15; HMS2051N19; HMS2098G15; HMS3413A19; HMS3677A19; HMS3715G15; FLUTICASONE PROPIONATE [MI]; AMY38235; HY-B0154; YMB56612; FLUTICASONE PROPIONATE [JAN]; Tox21_110698; AC-457; BDBM50354849; HB1091; s1992; FLUTICASONE PROPIONATE [VANDF]; AKOS015895220; Tox21_110698_1; CCG-100981; CS-1986; DB00588; FLUTICASONE PROPIONATE [WHO-DD]; KS-1173; NC00231; NCGC00179308-01; NCGC00179308-04; NCGC00179308-05; BF160362; BF161262; LS-19323; SMR000469159; FLUTICASONE PROPIONATE [ORANGE BOOK]; AB00513992; A51110; D01708; AB00513992-06; AB00513992_08; EN300-7480904; Fluticasone propionate - micronised pharma grade; Fluticasone propionate, >=98% (HPLC), powder; AN-584/43505443; SR-01000763355; Q-101393; Q8564098; SR-01000763355-3; BRD-K62310379-001-03-0; Fluticasone propionate 100 microg/mL in Acetonitrile; Fluticasone 17(2)-Carbonylsulfenic Acid 17-Propionate; Fluticasone propionate, European Pharmacopoeia (EP) Reference Standard; Fluticasone propionate, United States Pharmacopeia (USP) Reference Standard; Fluticasone Propionate, Pharmaceutical Secondary Standard; Certified Reference Material; (1R,2R,3aS,3bS,5S,9aS,9bR,10S,11aS)-5,9b-difluoro-1-{[(fluoromethyl)sulfanyl]carbonyl}-10-hydroxy-2,9a,11a-trimethyl-7-oxo-1H,2H,3H,3aH,3bH,4H,5H,7H,9aH,9bH,10H,11H,11aH-cyclopenta[a]phenanthren-1-yl propanoate; (1R,2S,8S,10S,11S,13R,14R,15S,17S)-1,8-difluoro-14-{[(fluoromethyl)sulfanyl]carbonyl}-17-hydroxy-2,13,15-trimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl propanoate; (6?,11?,16?,17?)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid fluoromethyl ester; (6alpha,11beta,16alpha,17alpha)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid fluoromethyl ester; (6alpha,11beta,16alpha,17alpha)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic Acid, S-(Fluoromethyl) Ester; ANDROSTA-1,4-DIENE-17-CARBOTHIOIC ACID, 6,9-DIFLUORO-11-HYDROXY-16-METHYL-3-OXO-17-(1-OXOPROPOXY)-, (6.ALPHA.,11.BETA.,16.ALPHA.,17.ALPHA.)-S-(FLUOROMETHYL) ESTER; Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, S-(fluoromethyl) ester, (6-alpha,11-beta,16-alpha,17-alpha)-; Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-,S-(fluoromethyl) ester, (6.alpha.,11.beta.,16.alpha.,17.alpha.)-; Fluticasone propionate for impurity C identification, EuropePharmacopoeia (EP) Reference Standard; Fluticasone propionate for impurity G identification, EuropePharmacopoeia (EP) Reference Standard; S-FLUOROMETHYL 6.ALPHA., 9.ALPHA.-DIFLUORO-11.BETA.-HYDROXY-16.ALPHA.-METHYL-3-OXO-17.ALPHA.-PROPIONYLOXYANDROSTA-1,4-DIENE-17.BETA-. CARBOTHIOATE; S-FLUOROMETHYL 6alpha, 9alpha-DIFLUORO-11beta-HYDROXY-16alpha-METHYL-3-OXO-17alpha-PROPIONYLOXYANDROSTA-1,4-DIENE-17.BETA-. CARBOTHIOATE; S-Fluoromethyl 6alpha,9alpha-difluoro-11beta-hydroxy-16alpha-methyl-3-oxo-17alpha-propionyloxyandrost-1,4-diene-17beta-carbothioate
Click to Show/Hide
|
|||||
| Target(s) | Glucocorticoid receptor (NR3C1) | |||||
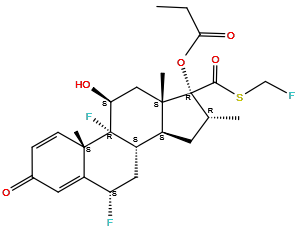
| Structure |
|
|||||
| Formula | C25H31F3O5S |
|||||
| Isosmiles | CCC(=O)O[C@@]1([C@@H](C[C@@H]2[C@@]1(C[C@@H]([C@]3([C@H]2C[C@@H](C4=CC(=O)C=C[C@@]43C)F)F)O)C)C)C(=O)SCF |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C25H31F3O5S/c1-5-20(31)33-25(21(32)34-12-26)13(2)8-15-16-10-18(27)17-9-14(29)6-7-22(17,3)24(16,28)19(30)11-23(15,25)4/h6-7,9,13,15-16,18-19,30H,5,8,10-12H2,1-4H3/t13-,15+,16+,18+,19+,22+,23+,24+,25+/m1/s1
|
|||||
| InChIKey |
WMWTYOKRWGGJOA-CENSZEJFSA-N
|
|||||
| IUPAC Name |
[(6S,8S,9R,10S,11S,13S,14S,16R,17R)-6,9-difluoro-17-(fluoromethylsulfanylcarbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate
|
|||||
| Pharmaceutical Properties | Molecule Weight |
500.6 |
Polar area |
106 |
||
Complexity |
984 |
xlogp Value |
4 |
|||
Heavy Count |
34 |
Rot Bonds |
6 |
|||
Hbond acc |
9 |
Hbond Donor |
1 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Maximal Effective Concentration (Emax) | 110 | % |
H4-II-E cells
|
Rat hepatocellular carcinoma
|
[1] | |
| Maximal Effective Concentration (Emax) | 113 | % |
Hep-G2 cells
|
Hepatoblastoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 3 | ug |
Neutrophil cells
|
Normal
|
Undisclosed | [2] |
| Maximal Effective Concentration (Emax) | 99 | % |
NCI-H292 cells
|
Lung mucoepidermoid carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.075 | nM |
NCI-H292 cells
|
Lung mucoepidermoid carcinoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.1 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [2] |
| Half Maximal Effective Concentration (EC50) | 0.1 | nM |
H4-II-E cells
|
Rat hepatocellular carcinoma
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 0.7 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 0.76 | nM |
H4-II-E cells
|
Rat hepatocellular carcinoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 1.09 | nM |
SW1353 cells
|
Bone chondrosarcoma
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | >19952.62 | nM |
Vero C1008 cells
|
Normal
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | >20000 | nM |
Vero C1008 cells
|
Normal
|
[4] | |
| Half Maximal Inhibitory Concentration (IC50) | 3290 | nM |
HCT-8 cells
|
Ileocecal adenocarcinoma
|
[5] |
Each Antibody-drug Conjugate Related to This Payload
References
