Payload Information
General Information of This Payload
| Payload ID | PAY0VXOJX |
|||||
|---|---|---|---|---|---|---|
| Name | GRM cpd 30 |
|||||
| Synonyms |
GRM cpd 30
Click to Show/Hide
|
|||||
| Target(s) | Glucocorticoid receptor (NR3C1) | |||||
| Structure |
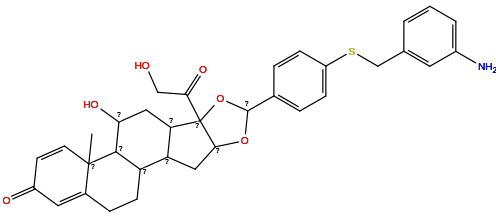
|
|||||
| Formula | C34H37NO6S |
|||||
| Isosmiles | CC12C=CC(=O)C=C1CCC1C3CC4OC(c5ccc(SCc6cccc(N)c6)cc5)OC4(C(=O)CO)C3CC(O)C12 |
|||||
| InChI |
InChI=1S/C34H37NO6S/c1-33-12-11-23(37)14-21(33)7-10-25-26-15-30-34(29(39)17-36,27(26)16-28(38)31(25)33)41-32(40-30)20-5-8-24(9-6-20)42-18-19-3-2-4-22(35)13-19/h2-6,8-9,11-14,25-28,30-32,36,38H,7,10,15-18,35H2,1H3
|
|||||
| InChIKey |
CWHLLYAJFSBWEI-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
587.738 |
Polar area |
119.08 |
||
Complexity |
42 |
xlogp Value |
4.7738 |
|||
Heavy Count |
42 |
Rot Bonds |
6 |
|||
Hbond acc |
8 |
Hbond Donor |
3 |
|||
The activity data of This Payload
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Anti-TNF ADC 129 [Investigative]
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Paw swelling AUC |
76.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 3 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Paw swelling AUC |
85.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
To evaluate the impact of the anti-mTNF GRM ADCs on inflammation in a chronic inflammatory setting,we progressed several ADCs into a mouse collagen-induced arthritis (mCIA) model. A single 10 mg/kg dose of the ADC was given at the first clinical signs of disease,a time point of intervention where anti-TNF treatment has a moderate impact.
|
||||
| In Vivo Model | Collagen-induced arthritis (mCIA) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | P1NP inhibition |
0.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | P1NP inhibition |
3.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Ear swelling inhibition |
73.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Ear swelling inhibition |
86.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Corticosterone inhibition |
7.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Corticosterone inhibition |
9.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.04 ug/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
50.00 ug/mL
|
Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
Anti-TNF ADC 115 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.03 ug/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.40 ug/mL
|
Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
References
