Payload Information
General Information of This Payload
| Payload ID | PAY0QBKLZ |
|||||
|---|---|---|---|---|---|---|
| Name | GRM cpd 26 |
|||||
| Synonyms |
GRM cpd 26
Click to Show/Hide
|
|||||
| Target(s) | Glucocorticoid receptor (NR3C1) | |||||
| Structure |
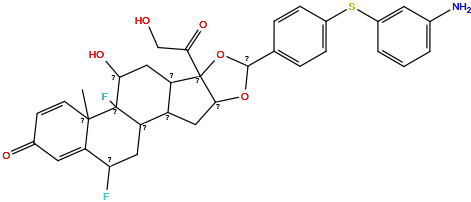
|
|||||
| Formula | C33H33F2NO6S |
|||||
| Isosmiles | CC12C=CC(=O)C=C1C(F)CC1C3CC4OC(c5ccc(Sc6cccc(N)c6)cc5)OC4(C(=O)CO)C3CC(O)C12F |
|||||
| InChI |
InChI=1S/C33H33F2NO6S/c1-31-10-9-19(38)12-25(31)26(34)14-24-22-13-29-32(28(40)16-37,23(22)15-27(39)33(24,31)35)42-30(41-29)17-5-7-20(8-6-17)43-21-4-2-3-18(36)11-21/h2-12,22-24,26-27,29-30,37,39H,13-16,36H2,1H3
|
|||||
| InChIKey |
VHFLAVGGDNGJPS-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties | Molecule Weight |
609.691 |
Polar area |
119.08 |
||
Complexity |
43 |
xlogp Value |
4.6728 |
|||
Heavy Count |
43 |
Rot Bonds |
5 |
|||
Hbond acc |
8 |
Hbond Donor |
3 |
|||
The activity data of This Payload
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
Anti-TNF ADC 128 [Investigative]
Obtained from the Model Organism Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | P1NP inhibition |
17.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | P1NP inhibition |
53.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess P1NP level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Ear swelling inhibition |
59.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Ear swelling inhibition |
92.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization.
|
||||
| In Vivo Model | Fluorescein isothiocyanate (FITC)-induced CHS model | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Corticosterone inhibition |
23.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 3 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Corticosterone inhibition |
74.00%
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
In an acute in vivo model of contact hypersensitivity (CHS) mice were sensitized with fluorescein isothiocyanate (FITC) on the abdomen and challenged 6 days later with FITC on the ear,which resulted in an increase in ear swelling that was measured 24 h postchallenge. Anti-mTNF GRM ADCs were dosed at either 10 mg/kg once prior to FITC sensitization. Mice were challenged with adrenocorticotropic hormone (ACTH) 72 h following ADC dosing and plasma was collected 30 min later to assess corticosterone level.
Click to Show/Hide
|
||||
| In Vivo Model | Acute contact hypersensitivity (CHS) model | ||||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.03 ug/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
3.90 ug/mL
|
Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
Anti-TNF ADC 114 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.12 ug/mL
|
Positive TNF expression (TNF+++/++) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells (TNF expression) | CVCL_0004 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 ug/mL | Negative TNF expression (TNF-) | ||
| Method Description |
All the DAR purified ADCs were screened in both the TNF-expressing and wild-type K562 GRE reporter cell assay to confirm that their activity was consistent with ADCs having heterogeneous average DAR.
|
||||
| In Vitro Model | Chronic myelogenous leukemia | K-562 cells | CVCL_0004 | ||
References
