Payload Information
General Information of This Payload
| Payload ID | PAY0NJQSP |
|||||
|---|---|---|---|---|---|---|
| Name | GNE-987 (R) |
|||||
| Synonyms |
GNE-987; 2417371-71-0; N-[11-({7-(3,5-difluoropyridin-2-yl)-2-methyl-10-[(methylsulfonyl)methyl]-3-oxo-3,4,6,7-tetrahydro-2H-2,4,7-triazadibenzo[cd,f]azulene-9-carbonyl}amino)undecanoyl]-3-methyl-L-valyl-(4R)-4-hydroxy-N-{[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}-L-prolinamide; 8-(3,5-difluoropyridin-2-yl)-N-[11-[[(2S)-1-[(2S,4R)-4-hydroxy-2-[[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methylcarbamoyl]pyrrolidin-1-yl]-3,3-dimethyl-1-oxobutan-2-yl]amino]-11-oxoundecyl]-15-methyl-4-(methylsulfonylmethyl)-14-oxo-8,12,15-triazatetracyclo[8.6.1.02,7.013,17]heptadeca-1(16),2(7),3,5,10,13(17)-hexaene-5-carboxamide; AKOS040733289; AT38265; HY-129937A; MS-31956; CS-0109237; EN300-33238021; (2S,4R)-1-[(2S)-2-(11-{[8-(3,5-difluoropyridin-2-yl)-4-(methanesulfonylmethyl)-15-methyl-14-oxo-8,12,15-triazatetracyclo[8.6.1.0^{2,7}.0^{13,17}]heptadeca-1(16),2,4,6,10,13(17)-hexaen-5-yl]formamido}undecanamido)-3,3-dimethylbutanoyl]-4-hydroxy-N-{[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}pyrrolidine-2-carboxamide; ;N-[11-({7-(3,5-difluoropyridin-2-yl)-2-methyl-10-[(methylsulfonyl)methyl]-3-oxo-3,4,6,7-tetrahydro-2H-2,4,7-triazadibenzo[cd,f]azulene-9-carbonyl}amino)undecanoyl]-3-methyl-L-valyl-(4R)-4-hydroxy-N-{[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}-L-prolinamide; WEP
Click to Show/Hide
|
|||||
| Target(s) | Bromodomain-containing protein 4 (BRD4) | |||||
| Structure |
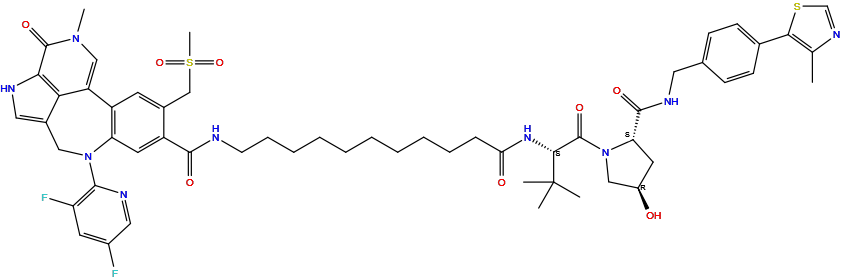
|
|||||
| Formula | C56H67F2N9O8S2 |
|||||
| Isosmiles | CC1=C(SC=N1)C2=CC=C(C=C2)CNC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)NC(=O)CCCCCCCCCCNC(=O)C4=CC5=C(C=C4CS(=O)(=O)C)C6=CN(C(=O)C7=C6C(=CN7)CN5C8=C(C=C(C=N8)F)F)C)O |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C56H67F2N9O8S2/c1-33-49(76-32-63-33)35-18-16-34(17-19-35)25-62-53(71)45-23-39(68)29-67(45)55(73)50(56(2,3)4)64-46(69)15-13-11-9-7-8-10-12-14-20-59-52(70)40-24-44-41(21-36(40)31-77(6,74)75)42-30-65(5)54(72)48-47(42)37(26-60-48)28-66(44)51-43(58)22-38(57)27-61-51/h16-19,21-22,24,26-27,30,32,39,45,50,60,68H,7-15,20,23,25,28-29,31H2,1-6H3,(H,59,70)(H,62,71)(H,64,69)/t39-,45+,50-/m1/s1
|
|||||
| InChIKey |
VTPSYVSGGUUAFN-GDNJTPAESA-N
|
|||||
| IUPAC Name |
8-(3,5-difluoropyridin-2-yl)-N-[11-[[(2S)-1-[(2S,4R)-4-hydroxy-2-[[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methylcarbamoyl]pyrrolidin-1-yl]-3,3-dimethyl-1-oxobutan-2-yl]amino]-11-oxoundecyl]-15-methyl-4-(methylsulfonylmethyl)-14-oxo-8,12,15-triazatetracyclo[8.6.1.02,7.013,17]heptadeca-1(16),2(7),3,5,10,13(17)-hexaene-5-carboxamide
|
|||||
| Pharmaceutical Properties | Molecule Weight |
1096.3 |
Polar area |
264 |
||
Complexity |
2210 |
xlogp Value |
6.8 |
|||
Heavy Count |
77 |
Rot Bonds |
22 |
|||
Hbond acc |
14 |
Hbond Donor |
5 |
|||
Each Antibody-drug Conjugate Related to This Payload
Full Information of The Activity Data of The ADC(s) Related to This Payload
CLL1-5 ADC [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 27.70% (Day 6) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing EOL-1 AML xenografts, each conjugate (in 0.5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 48.81% (Day 10) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing HL-60 AML xenografts, each conjugate (in 0.5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 61.20% (Day 6) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing EOL-1 AML xenografts, each conjugate (in 1.5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 71.08% (Day 10) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing HL-60 AML xenografts, each conjugate (in 1.5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 79.20% (Day 6) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing EOL-1 AML xenografts, each conjugate (in 5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 89.20% (Day 6) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing EOL-1 AML xenografts, each conjugate (in 10 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | EOL-1 CDX model | ||||
| In Vitro Model | Chronic eosinophilic leukemia | EoL-1 cells | CVCL_0258 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 93.89% (Day 10) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing HL-60 AML xenografts, each conjugate (in 5 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 10) | High CCL1 expression (CCL1 +++) | ||
| Method Description |
Single intravenous (IV) administration of the CLL1-5 ADC to mice bearing HL-60 AML xenografts, each conjugate (in 10 mg/kg) that were administered once IV at the day 0 time point.
|
||||
| In Vivo Model | HL-60 CDX model | ||||
| In Vitro Model | Adult acute myeloid leukemia | HL-60 cells | CVCL_0002 | ||
References
