Payload Information
General Information of This Payload
| Payload ID | PAY0KYMRL |
|||||
|---|---|---|---|---|---|---|
| Name | Dexamethasone |
|||||
| Synonyms |
Dexamethasone; 50-02-2; Decadron; Maxidex; Dexamethazone; Hexadecadrol; Millicorten; Decaspray; Dexasone; Hexadrol; Oradexon; Prednisolone F; Aeroseb-Dex; Fluormethylprednisolone; Desametasone; Superprednol; Visumetazone; Cortisumman; Decaderm; Dexacortal; Dexacortin; Fortecortin; Gammacorten; Auxiron; Calonat; Dexason; Dexone; Azium; Aphtasolon; Dectancyl; Deltafluorene; Dergramin; Desadrene; Desamethasone; Desameton; Dexadeltone; Dexapolcort; Mediamethasone; Mymethasone; Corsone; Decalix; Decasone; Dekacort; Dexacort; Dexalona; Dexameth; Dextelan; Dinormon; Loverine; Luxazone; Mexidex; Dexa-Cortidelt; Dexa-Scheroson; Dexa-Cortisyl; Prednisolon F; Dexa-sine; Isopto-Dex; SK-Dexamethasone; Aeroseb-D; Anaflogistico; Decacortin; Deseronil; Dexafarma; Dexaprol; Dexinolon; Dexinoral; Policort; Spoloven; Turbinaire; Deronil; Dexapos; Sunia Sol D; Bisu DS; Dexa Mamallet; Lokalison F; Ocu-trol; Dex-ide; Hl-dex; Dexamethasonum; Decameth; Fluormone; Fluorocort; Decagel; Dexpak; Dezone; Dexone 4; Dxms; Hexadrol Elixir; Pet Derm III; Dexamethasone alcohol; Dexamethasone Intensol; Methylfluorprednisolone; Dexone 0.5; Dexone 1.5; Dexametasona; Decaject; Dexone 0.75; Hexadrol Tablets; Desametasone [DCIT]; Aphthasolone; Decacort; Dexamonozon; Dextenza; Ozurdex; 9alpha-Fluoro-16alpha-methylprednisolone; Dexametasona [INN-Spanish]; Dexamethasonum [INN-Latin]; Decadron Tablets, Elixir; Decaject-L.A.; Azium (Veterinary); Dexametasone; Dexamethansone; MK 125; Adexone; Dexaltin; Osurdex; Posurdex; Dexycu; Prodex; DexaSite; OTO-104; Dexa-Mamallet; Corson; NSC 34521; 16alpha-Methyl-9alpha-fluoro-1-dehydrocortisol; 16alpha-Methyl-9alpha-fluoroprednisolone; (3H)-Dexamethasone; 16-alpha-Methyl-9-alpha-fluoroprednisolone; 9-alpha-Fluoro-16-alpha-methylprednisolone; Azimycin (Veterinary); delta1-9alpha-Fluoro-16alpha-methylcortisol; 1-Dehydro-16alpha-methyl-9alpha-fluorohydrocortisone; Naquasone (Veterinary); Tresaderm (Veterinary); CCRIS 7067; Dexonium; Diodex; DTXSID3020384; HSDB 3053; ISV-305; 16-alpha-Methyl-9-alpha-fluoro-1-dehydrocortisol; Zema-Pak; UNII-7S5I7G3JQL; Dexamethasone Base; 16-alpha-Methyl-9-alpha-fluoro-delta1-hydrocortisone; delta(sup 1)-9-alpha-Fluoro-16-alpha-methylcortisol; EINECS 200-003-9; 7S5I7G3JQL; MFCD00064136; NSC-34521; Decadron (TN); 16alpha-Methyl-9alpha-fluoro-delta(sup 1)-hydrocortisone; DXM [Steroid]; 16-alpha-Methyl-9-alpha-fluoro-delta(sup 1)-hydrocortisone; HEMADY; Dexamethasone [INN:BAN:JAN]; Dexamethasone (GMP); Dexamethasone, topical; CHEBI:41879; AI3-50934; 9A-FLUORO-16BETA-METHYLPREDNISOLONE; Prednisolone, 9alpha-fluoro-16alpha-methyl-; C22H29FO5; (11beta,16alpha)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; Dexamethasone (DHAP); dexmethsone; DTXCID10384; MLS001332507; 16.alpha.-Methyl-9.alpha.-fluoroprednisolone; Dexamethasone [USP:INN:BAN:JAN]; DXM (Steroid); 9-Fluoro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione; [3H]-dexamethasone; Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11beta,16alpha)-; CIPRODEX COMPONENT DEXAMETHASONE; MAXITROL COMPONENT DEXAMETHASONE; TOBRADEX COMPONENT DEXAMETHASONE; (1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,13,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one; DEXACIDIN COMPONENT DEXAMETHASONE; DEXASPORIN COMPONENT DEXAMETHASONE; DEXAMETHASONE COMPONENT OF CIPRODEX; DEXAMETHASONE COMPONENT OF MAXITROL; DEXAMETHASONE COMPONENT OF TOBRADEX; TOBRADEX ST COMPONENT DEXAMETHASONE; DEXAMETHASONE COMPONENT OF DEXACIDIN; DEXAMETHASONE COMPONENT OF DEXASPORIN; DEXAMETHASONE COMPONENT OF TOBRADEX ST; 9.alpha.-Fluoro-16.alpha.-methylprednisolone; 16-alpha-Methyl-9-alpha-fluoro-1,4-pregnadiene-11-beta,17-alpha,21-triol-3,20-dione; 16-alpha-Methyl-9-alpha-fluoro-11-beta,17-alpha,21-trihydroxypregna-1,4-diene-3,20-dione; 4-alpha-Fluoro-16-alpha-methyl-11-beta,17,21-trihydroxypregna-1,4-diene-3,20-dione; 9-alpha-Fluoro-16-alpha-methyl-1,4-pregnadiene-11-beta,17-alpha,21-triol-3,20-dione; (8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; (8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one; DEXAMETHASONE (MART.); DEXAMETHASONE [MART.]; 16.alpha.-Methyl-9.alpha.-fluoro-1-dehydrocortisol; 1-Dehydro-16-alpha-methyl-9-alpha-fluorohydrocortisone; 1-Dehydro-16.alpha.-methyl-9.alpha.-fluorohydrocortisone; IontoDex; DEXAMETHASONE (EP MONOGRAPH); DEXAMETHASONE [EP MONOGRAPH]; DEXAMETHASONE (USP MONOGRAPH); DEXAMETHASONE [USP MONOGRAPH]; Pet-Derm Iii; Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17,21-trihydroxy-16alpha-methyl-; Decaject L.A.; (11beta,16alpha)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione labeled with tritium; 23495-06-9; Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, labeled with tritium, (11beta,16alpha)-; SMR000857119; BETAMETHASONE IMPURITY A (EP IMPURITY); BETAMETHASONE IMPURITY A [EP IMPURITY]; dexamethasone (tetramethyl-rhodamine conjugated ); Prednisolone, 9.alpha.-fluoro-16.alpha.-methyl-; Amplidermis; Auricularum; Cortidexason; Dexafluorene; Dexamecortin; Fluorodelta; Aacidexam; Baycadron; Decadrol; Dexalocal; Dexasine; Solurex; Spersadex; .delta.(sup 1)-9-.alpha.-Fluoro-16-.alpha.-methylcortisol; 16.alpha.-Methyl-9.alpha.-fluoro-.delta.(sup1)-hydrocortisone; Dxevo; NSC34521; TaperDex; Aknichthol Dexa; Alin Oftalmico; Anemul mono; Dexa-Rhinosan; .gamma.corten; ZoDex; 9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; Alin Depot; Baycuten N; C22-H29-F-O5; Lokalison-F; Apo-dexamethasone; Decadron DP; Dexasone 4mg; Alba-Dex; PHL-dexamethasone; PMS-dexamethasone; [3H]dexamethasone; Decasone R.p.; Dexamethasone-omega; NCGC00091019-08; Dexasone 0.5mg; Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17,21-trihydroxy-16-methyl-, (11.beta.,16.alpha.)-; Dexasone 0.75mg; Sandoz Dexamethasone; DEXASCHEROSON; DEXYCU KIT; Maxidex Ont 0.1%; Maxidex Sus 0.1%; Decadron, Dexamethasone; dexamethasone-17-acetate; Spectrum5_002019; DEXAMETHASONE [MI]; D0IT2G; Foy Brand of Dexamethasone; MolMap_000018; DEXAMETHASONE [INN]; DEXAMETHASONE [JAN]; ECR Brand of Dexamethasone; ICN Brand of Dexamethasone; SCHEMBL3774; Alcon Brand of Dexamethasone; DEXAMETHASONE [HSDB]; Merck Brand of Dexamethasone; BIDD:PXR0060; BSPBio_000995; DEXAMETHASONE [VANDF]; MLS001055412; MLS001332508; BIDD:ER0494; sustained-release dexamethasone; DEXAMETHASONE [USP-RS]; DEXAMETHASONE [WHO-DD]; DEXAMETHASONE [WHO-IP]; Merz Brand 1 of Dexamethasone; Merz Brand 2 of Dexamethasone; 3,20-DIONE; CHEMBL384467; Dexamethasone [BAN:INN:JAN]; GTPL2768; GTPL3447; SGCUT00126; 9-Fluoro-16-methylprednisolone; Ak Dex Oph Otic Soln 0.1%; DEXAMETHASONE [EMA EPAR]; BDBM18207; Dexamethasone (anti-inflammatory); Dexamethasone (JP15/USP/INN); Dexamethasone (JP17/USP/INN); (11-beta,16-alpha)-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione; 1p93; DEXAMETHASONE [GREEN BOOK]; HMS1792A17; HMS1990A17; HMS2089N13; HMS2235F08; HMS3039L11; HMS3259N11; HMS3403A17; DEXAMETHASONE [ORANGE BOOK]; AMY28815; DRG-0013; to_000038; Tox21_200122; Dexamethasone, >=97.0% (HPLC); HB2521; HY-14648G; IBI-10090; MK-125; s1322; Dexamethasone 1.0 mg/ml in Methanol; DEXAMETHASONUM [WHO-IP LATIN]; PMS Dexamethasone Elixir 0.5mg/5ml; AKOS005259009; AKOS015895509; Decadron phosphate, Decdan, Dexacort,; CCG-264887; CS-1505; CS-O-00294; CS-O-01282; CS-O-01283; DB01234; KS-1451; LS-7300; NC00645; alpha -Fluoro-16-alpha -methylcortisol; CAS-50-02-2; SMP1_000092; Dexamethasone, >=98% (HPLC), powder; NCGC00091019-01; NCGC00091019-02; NCGC00091019-03; NCGC00091019-04; NCGC00091019-05; NCGC00091019-06; NCGC00091019-07; NCGC00091019-23; NCGC00257676-01; (8S,9R,10S,11S,13S,14S,16R,17R)-9-Fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]ph; 9alpha-fluoro-16alpha-methyl-Prednisolone; AC-11056; HY-14648; NCI60_003067; SMR001227192; Fluoro-9alpha Methyl-16alpha Prednisolone; 16alpha -Methyl-9alpha -fluoroprednisolone; 9alpha -Fluoro-16alpha -methylprednisolone; Dexamethasone, tested according to Ph.Eur.; CS-0626118; EN300-52607; D00292; Dexamethasone, meets USP testing specifications; Prednisolone, 9alpha -fluoro-16alpha -methyl-; 9.alpha.-Fluoro-16.alpha.-methyl-1,20-dione; AB00918428-05; AB00918428-08; AB00918428-09; AB00918428_10; 16alpha -Methyl-9alpha -fluoro-1-dehydrocortisol; 16Alpha-methyl-9alpha-fluoro-delta1-hydrocortisone; Dexamethasone, VETRANAL(TM), analytical standard; Q422252; 1-Dehydro-16alpha-methyl-0alpha-fluorohydrocortisone; delta(Sup1)-9-alpha-fluoro-16-alpha-methylcortisol; Q-200939; 16-alpha-methyl-9-alpha-fluoro-delta-1-hydrocortisone; BRD-K38775274-001-02-3; BRD-K38775274-001-06-4; DEXAMETHASONE ACETATE IMPURITY A [EP IMPURITY]; 1-Dehydro-16alpha -methyl-9alpha -fluorohydrocortisone; 9-Fluoro-11alpha -methylpregna-1,4-diene-3,20-dione; Dexamethasone, British Pharmacopoeia (BP) Assay Standard; Z756391748; Pregna-1,4-diene-3,20-dione, 9-fluoro-11alpha -methyl-; Dexamethasone, European Pharmacopoeia (EP) Reference Standard; WLN: L E5 B666 OV KU MUTJ A1 BF CQ E1 FV1Q FQ G1; 16-ALPHA-METHYL-9-ALPHA-FLUORO-DELTA(SUP 1)HYDROCORTISONE; Dexamethasone, powder, BioReagent, suitable for cell culture, >=97%; Dexamethasone, United States Pharmacopeia (USP) Reference Standard; 16-.alpha.-Methyl-9-.alpha.-fluoro-1,17-.alpha.,21-triol-3,20-dione; 4.alpha.-Fluoro-16.alpha.-methyl-11.beta.-17,4-diene-3,20-dione; Pregna-1,20-dione, 9-fluoro-11.beta.,17,21-trihydroxy-16.alpha.-methyl-; 16.alpha.-Methyl-9.alpha.-fluoro-11.beta.-17.alpha.-21-trihydroxypregna-1,20-dione; 9-Fluoro-11-beta,17,21-trihydroxy-16-alpha-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11.beta.,21-trihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione; 9-fluoro-16alpha-methyl-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione; 9.alpha.-Fluoro-11.beta.-17.alpha.- 21-trihydroxy-16.alpha.-methylpregna-1,20-dione; Dexamethasone for peak identification, European Pharmacopoeia (EP) Reference Standard; Dexamethasone for system suitability, European Pharmacopoeia (EP) Reference Standard; Dexamethasone, Pharmaceutical Secondary Standard; Certified Reference Material; Dexamethasone, powder, gamma-irradiated, BioXtra, suita
Click to Show/Hide
|
|||||
| Target(s) | Glucocorticoid receptor (NR3C1) | |||||
| Structure |
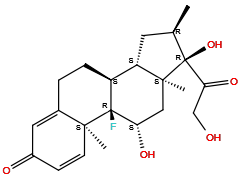
|
|||||
| Formula | C22H29FO5 |
|||||
| Isosmiles | C[C@@H]1C[C@H]2[C@@H]3CCC4=CC(=O)C=C[C@@]4([C@]3([C@H](C[C@@]2([C@]1(C(=O)CO)O)C)O)F)C |
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
|
|||||
| InChIKey |
UREBDLICKHMUKA-CXSFZGCWSA-N
|
|||||
| IUPAC Name |
(8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
|
|||||
| Pharmaceutical Properties | Molecule Weight |
392.5 |
Polar area |
94.8 |
||
Complexity |
805 |
xlogp Value |
1.9 |
|||
Heavy Count |
28 |
Rot Bonds |
2 |
|||
Hbond acc |
6 |
Hbond Donor |
3 |
|||
The activity data of This Payload
| Standard Type | Value | Units | Cell line | Disease Model | Cell line ID | Reference |
|---|---|---|---|---|---|---|
| Half Maximal Inhibitory Concentration (IC50) | 0.016 | ug/mL |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 0.165 | ug/mL |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.84 | ug/mL |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[1] | |
| Half Maximal Inhibitory Concentration (IC50) | >25 | ug/mL |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 36 | ug/mL |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[2] | |
| Half Maximal Inhibitory Concentration (IC50) | 9.09 | ug/mL |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[1] | |
| Half Maximal Effective Concentration (EC50) | 0.2 | nM |
CV-1 cells
|
Normal
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 0.47 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 0.5 | nM |
Human foreskin fibroblasts cells
|
Normal
|
Undisclosed | [5] |
| Half Maximal Inhibitory Concentration (IC50) | 0.5082 | nM |
U2OS cells
|
Osteosarcoma
|
[6] | |
| Half Maximal Effective Concentration (EC50) | 0.92 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[7] | |
| Half Maximal Inhibitory Concentration (IC50) | 1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | 1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[9] | |
| Half Maximal Inhibitory Concentration (IC50) | 1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[10] | |
| Half Maximal Inhibitory Concentration (IC50) | 1 | nM |
Human foreskin fibroblasts cells
|
Normal
|
Undisclosed | [11] |
| Half Maximal Effective Concentration (EC50) | 1 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 1 | nM |
Splenocyte cells
|
Normal
|
Undisclosed | [12] |
| Half Maximal Effective Concentration (EC50) | 1.1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[13] | |
| Half Maximal Effective Concentration (EC50) | 1.1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[14] | |
| Half Maximal Effective Concentration (EC50) | 1.1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[15] | |
| Half Maximal Effective Concentration (EC50) | 1.1 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.175 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[17] | |
| Half Maximal Effective Concentration (EC50) | 1.2 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 1.259 | nM |
INS-1 832/13 cells
|
Rat insulinoma
|
[18] | |
| Half Maximal Effective Concentration (EC50) | 1.3 | nM |
HEK293 cells
|
Normal
|
[19] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.585 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.585 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 1.8 | nM |
SW1353 cells
|
Bone chondrosarcoma
|
[22] | |
| Half Maximal Effective Concentration (EC50) | 1.9 | nM |
Human foreskin fibroblasts cells
|
Normal
|
Undisclosed | [23] |
| Half Maximal Effective Concentration (EC50) | 1.9 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 1.995 | nM |
INS-1 832/13 cells
|
Rat insulinoma
|
[24] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 10 | nM |
SN12C cells
|
Renal cell carcinoma
|
[25] | |
| Half Maximal Effective Concentration (EC50) | 10 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[27] | |
| Half Maximal Inhibitory Concentration (IC50) | 10 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[28] | |
| Half Maximal Inhibitory Concentration (IC50) | <1000 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[8] | |
| Half Maximal Inhibitory Concentration (IC50) | >1000 | nM |
Sf9 cells
|
Normal
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | >1000 | nM |
Sf9 cells
|
Normal
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | >1000 | nM |
Sf9 cells
|
Normal
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | <10000 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[29] | |
| Half Maximal Inhibitory Concentration (IC50) | >10000 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[17] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HL-60 cells
|
Adult acute myeloid leukemia
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SW620 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
K-562 cells
|
Chronic myelogenous leukemia
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | >100000 | nM |
THP-1 cells
|
Childhood acute monocytic leukemia
|
[30] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
U-251MG cells
|
Astrocytoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SK-MEL-2 cells (MEK inhibitor-resistant)
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
COLO 205 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
Caki-1 cells
|
Clear cell renal cell carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
DLD-1 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HCT 116 cells
|
Colon carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HCT 15 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HT-29 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
OVCAR-3 cells (FZD7 overexpression)
|
Ovarian serous adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SK-MEL-28 cells (BRAF inhibitor resistant)
|
Cutaneous melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SK-MEL-5 cells
|
Cutaneous melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SNB-19 cells
|
Astrocytoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
786-O cells
|
Renal cell carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
IGROV-1 cells
|
Ovarian endometrioid adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
NCI-H226 cells
|
Pleural epithelioid mesothelioma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
NCI-H23 cells
|
Lung adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
NCI-H322M cells
|
Minimally invasive lung adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
OVCAR-5 cells
|
Ovarian serous adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
OVCAR-8 cells
|
High grade ovarian serous adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SF539 cells
|
Gliosarcoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SNB-75 cells
|
Glioblastoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SK-OV-3 cells (FZD7 overexpression)
|
Ovarian serous cystadenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SF268 cells
|
Astrocytoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | >100000 | nM |
Multiple myeloma cancer stem cells
|
Multiple myeloma
|
Undisclosed | [31] |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
ACHN cells
|
Renal adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HOP-62 cells
|
Non-small cell lung carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
HOP-92 cells
|
Non-small cell lung carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
KM12 cells
|
Colon adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
M14 cells
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
Malme-3M cells
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
NCI-H522 cells
|
Non-small cell lung carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
OVCAR-4 cells
|
Ovarian adenocarcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SF-295 cells
|
Glioblastoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
TK-10 cells
|
Renal carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
UACC-257 cells
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
UACC-62 cells
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
UO-31 cells
|
Renal carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
XF498 cells
|
Glioma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
SNB-78 cells
|
Glioblastoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
M19-MEL cells
|
Melanoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
LXFL 529 cells
|
Non-small cell lung carcinoma
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 100000 | nM |
KM-20L2 cells
|
Colon carcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 10700 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[32] | |
| Half Maximal Inhibitory Concentration (IC50) | 12.59 | nM |
Sf9 cells
|
Normal
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | 1200 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[33] | |
| Half Maximal Effective Concentration (EC50) | 130 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 130 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[34] | |
| Half Maximal Inhibitory Concentration (IC50) | 134 | nM |
Macrophage cells
|
Normal
|
Undisclosed | [35] |
| Half Maximal Inhibitory Concentration (IC50) | 137400 | nM |
Sf21 cells
|
Normal
|
[36] | |
| Half Maximal Effective Concentration (EC50) | 14 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 1430 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[37] | |
| Half Maximal Inhibitory Concentration (IC50) | 14750 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[38] | |
| Half Maximal Inhibitory Concentration (IC50) | 150 | nM |
Peritoneal macrophage cells
|
Normal
|
Undisclosed | [39] |
| Half Maximal Inhibitory Concentration (IC50) | >15000 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[40] | |
| Half Maximal Inhibitory Concentration (IC50) | >15000 | nM |
MCF7-F (fulvestrant resistant) cells
|
Invasive breast carcinoma
|
[40] | |
| Half Maximal Inhibitory Concentration (IC50) | >15000 | nM |
Fibroblast cells
|
Normal
|
Undisclosed | [40] |
| Half Maximal Effective Concentration (EC50) | <15848.93 | nM |
U2OS cells
|
Osteosarcoma
|
[6] | |
| Half Maximal Inhibitory Concentration (IC50) | 159200 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[41] | |
| Half Maximal Inhibitory Concentration (IC50) | 159200 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[42] | |
| Half Maximal Inhibitory Concentration (IC50) | 1600 | nM |
T-cells
|
Normal
|
Undisclosed | [43] |
| Half Maximal Inhibitory Concentration (IC50) | 169500 | nM |
J774 cells
|
Mouse reticulum cell sarcoma
|
[44] | |
| Half Maximal Effective Concentration (EC50) | 17 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[23] | |
| Half Maximal Effective Concentration (EC50) | 17 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[45] | |
| Half Maximal Effective Concentration (EC50) | 17 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[46] | |
| Half Maximal Inhibitory Concentration (IC50) | 17 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [47] |
| Half Maximal Inhibitory Concentration (IC50) | 170000 | nM |
J774.1 cells
|
Reticulum cell sarcoma
|
[48] | |
| Half Maximal Inhibitory Concentration (IC50) | 18400 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[49] | |
| Half Maximal Inhibitory Concentration (IC50) | >19952.62 | nM |
Vero C1008 cells
|
Normal
|
[50] | |
| Half Maximal Effective Concentration (EC50) | 2 | nM |
Human foreskin fibroblasts cells
|
Normal
|
Undisclosed | [5] |
| Half Maximal Inhibitory Concentration (IC50) | 2 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [51] |
| Half Maximal Effective Concentration (EC50) | 2.1 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 2.3 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [52] |
| Half Maximal Effective Concentration (EC50) | 2.4 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 2.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[13] | |
| Half Maximal Effective Concentration (EC50) | 2.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[14] | |
| Half Maximal Effective Concentration (EC50) | 2.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[15] | |
| Half Maximal Effective Concentration (EC50) | 2.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[16] | |
| Half Maximal Effective Concentration (EC50) | 2.5 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[53] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.512 | nM |
THP-1 cells
|
Childhood acute monocytic leukemia
|
[20] | |
| Half Maximal Inhibitory Concentration (IC50) | 2.512 | nM |
THP-1 cells
|
Childhood acute monocytic leukemia
|
[52] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 20 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[54] | |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
RAW cells
|
Monocytic-macrophage leukemia
|
Undisclosed | [55] |
| Half Maximal Inhibitory Concentration (IC50) | 20 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[56] | |
| Half Maximal Effective Concentration (EC50) | >200 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | >20000 | nM |
B16-F10 cells
|
Mouse melanoma
|
[40] | |
| Half Maximal Inhibitory Concentration (IC50) | >20000 | nM |
HEK-293T cells
|
Normal
|
[40] | |
| Half Maximal Inhibitory Concentration (IC50) | >20000 | nM |
CHO cells
|
Normal
|
[40] | |
| Half Maximal Inhibitory Concentration (IC50) | >20000 | nM |
Vero C1008 cells
|
Normal
|
[50] | |
| Half Maximal Inhibitory Concentration (IC50) | 22000 | nM |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[57] | |
| Half Maximal Inhibitory Concentration (IC50) | 22200 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[58] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 23.93 | nM |
CCRF-CEM cells
|
T acute lymphoblastic leukemia
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 231900 | nM |
Sf21 cells
|
Normal
|
[36] | |
| Half Maximal Inhibitory Concentration (IC50) | 24300 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[59] | |
| Half Maximal Inhibitory Concentration (IC50) | 25 | nM |
BV-2 cells
|
Normal
|
[60] | |
| Half Maximal Inhibitory Concentration (IC50) | 2500 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[61] | |
| Half Maximal Effective Concentration (EC50) | 2600 | nM |
Hep-G2 cells
|
Hepatoblastoma
|
[62] | |
| Half Maximal Inhibitory Concentration (IC50) | 2660 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[63] | |
| Half Maximal Effective Concentration (EC50) | 3.162 | nM |
INS-1 832/13 cells
|
Rat insulinoma
|
[18] | |
| Half Maximal Effective Concentration (EC50) | 3.5 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 3.6 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 3.6 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [64] |
| Half Maximal Inhibitory Concentration (IC50) | 3.8 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [52] |
| Half Maximal Inhibitory Concentration (IC50) | 3.8 | nM |
IM-9 cells
|
Multiple myeloma
|
[65] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.8 | nM |
IM-9 cells
|
Multiple myeloma
|
[66] | |
| Half Maximal Inhibitory Concentration (IC50) | 3.981 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[10] | |
| Half Maximal Effective Concentration (EC50) | 30 | nM |
Farage cells
|
Normal
|
[67] | |
| Half Maximal Inhibitory Concentration (IC50) | 32500 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[68] | |
| Half Maximal Inhibitory Concentration (IC50) | 355140 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[69] | |
| Half Maximal Effective Concentration (EC50) | 4.1 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 4.1 | nM |
HeLa S3 cells
|
Endocervical adenocarcinoma
|
[70] | |
| Half Maximal Effective Concentration (EC50) | 4.2 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[15] | |
| Half Maximal Effective Concentration (EC50) | 4.2 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[53] | |
| Half Maximal Effective Concentration (EC50) | 4.2 | nM |
NP1 cells
|
Normal
|
[13] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.3 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [52] |
| Half Maximal Effective Concentration (EC50) | 4.5 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[14] | |
| Half Maximal Effective Concentration (EC50) | 4.5 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[16] | |
| Half Maximal Inhibitory Concentration (IC50) | 4.6 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [52] |
| Half Maximal Inhibitory Concentration (IC50) | 40 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[57] | |
| Half Maximal Inhibitory Concentration (IC50) | 400 | nM |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[57] | |
| Half Maximal Inhibitory Concentration (IC50) | 4160 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[71] | |
| Half Maximal Inhibitory Concentration (IC50) | 420 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[72] | |
| Half Maximal Inhibitory Concentration (IC50) | 4620580 | nM |
L02 cells
|
Cervical carcinoma
|
[73] | |
| Half Maximal Inhibitory Concentration (IC50) | 4620580 | nM |
L02 cells
|
Cervical carcinoma
|
[73] | |
| Half Maximal Inhibitory Concentration (IC50) | 470 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[74] | |
| Half Maximal Inhibitory Concentration (IC50) | 4700 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[57] | |
| Half Maximal Inhibitory Concentration (IC50) | 48 | nM |
J774.A1 cells
|
Mouse reticulum cell sarcoma
|
[75] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 49317.38 | nM |
SR cells
|
Leukemia
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
T-cells
|
Normal
|
Undisclosed | [76] |
| Half Maximal Inhibitory Concentration (IC50) | 5 | nM |
R1 cells
|
Normal
|
[77] | |
| Half Maximal Effective Concentration (EC50) | 5.012 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[17] | |
| Half Maximal Effective Concentration (EC50) | 5.37 | nM |
A-549 cells
|
Lung adenocarcinoma
|
[8] | |
| Half Maximal Effective Concentration (EC50) | 5.5 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 50 | nM |
THP-1 cells
|
Childhood acute monocytic leukemia
|
[30] | |
| Half Maximal Inhibitory Concentration (IC50) | >50000 | nM |
LLC-PK1 cells
|
Normal
|
[78] | |
| Half Maximal Inhibitory Concentration (IC50) | >50000 | nM |
LLC-PK1 cells
|
Normal
|
[78] | |
| Half Maximal Inhibitory Concentration (IC50) | >50000 | nM |
LLC-PK1 cells
|
Normal
|
[78] | |
| Half Maximal Inhibitory Concentration (IC50) | >50000 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[73] | |
| Half Maximal Inhibitory Concentration (IC50) | >50000 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[73] | |
| Half Maximal Inhibitory Concentration (IC50) | >500000 | nM |
HEK293 cells
|
Normal
|
[79] | |
| Half Maximal Inhibitory Concentration (IC50) | 5020 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[41] | |
| Half Maximal Inhibitory Concentration (IC50) | 5020 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[42] | |
| Half Maximal Inhibitory Concentration (IC50) | 5200 | nM |
J774 cells
|
Mouse reticulum cell sarcoma
|
[80] | |
| Half Maximal Effective Concentration (EC50) | 55 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 5500 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[81] | |
| Half Maximal Inhibitory Concentration (IC50) | 580 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[82] | |
| Half Maximal Effective Concentration (EC50) | 6.1 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 6.31 | nM |
INS-1 832/13 cells
|
Rat insulinoma
|
[24] | |
| Half Maximal Inhibitory Concentration (IC50) | 6.4 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [52] |
| Half Maximal Inhibitory Concentration (IC50) | 6.5 | nM |
RPMI-8226 cells
|
Plasma cell myeloma
|
[3] | |
| Half Maximal Effective Concentration (EC50) | 6.7 | nM |
Reh cells
|
B acute lymphoblastic leukemia
|
[83] | |
| Half Maximal Inhibitory Concentration (IC50) | 6000 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[84] | |
| Half Maximal Inhibitory Concentration (IC50) | 6100 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[84] | |
| Half Maximal Inhibitory Concentration (IC50) | 630 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[85] | |
| Half Maximal Inhibitory Concentration (IC50) | 630 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[86] | |
| Half Maximal Effective Concentration (EC50) | 66 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 6900 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[87] | |
| Half Maximal Inhibitory Concentration (IC50) | 7 | nM |
THP-1 cells
|
Childhood acute monocytic leukemia
|
[30] | |
| Half Maximal Effective Concentration (EC50) | 7.2 | nM |
COS-7 cells
|
Normal
|
[88] | |
| Half Maximal Effective Concentration (EC50) | 7.6 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Effective Concentration (EC50) | 7.943 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[20] | |
| Half Maximal Effective Concentration (EC50) | 7.943 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[52] | |
| Half Maximal Inhibitory Concentration (IC50) | 7.943 | nM |
HeLa cells
|
Endocervical adenocarcinoma
|
[21] | |
| Half Maximal Inhibitory Concentration (IC50) | 700 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[89] | |
| Half Maximal Inhibitory Concentration (IC50) | 7000 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[90] | |
| Half Maximal Effective Concentration (EC50) | 72 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 74 | nM |
N9 cells
|
Normal
|
Undisclosed | [72] |
| Half Maximal Inhibitory Concentration (IC50) | 7900 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[91] | |
| Half Maximal Inhibitory Concentration (IC50) | 7900 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[92] | |
| Half Maximal Inhibitory Concentration (IC50) | 8 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [51] |
| Half Maximal Inhibitory Concentration (IC50) | 8.06 | nM |
HeLa S3 cells
|
Endocervical adenocarcinoma
|
[93] | |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
B-cells
|
Normal
|
Undisclosed | [43] |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[26] | |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[94] | |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[95] | |
| Half Maximal Inhibitory Concentration (IC50) | 800 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[96] | |
| Half Maximal Effective Concentration (EC50) | 82 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 8280 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[97] | |
| Half Maximal Inhibitory Concentration (IC50) | 8300 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[98] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 83560.3 | nM |
EKVX cells
|
Non-small cell lung carcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 8400 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[84] | |
| Half Maximal Inhibitory Concentration (IC50) | 860 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[99] | |
| Half Maximal Inhibitory Concentration (IC50) | 8700 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[100] | |
| Half Maximal Inhibitory Concentration (IC50) | 8710 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[73] | |
| Half Maximal Effective Concentration (EC50) | 88 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 9 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [51] |
| Half Maximal Effective Concentration (EC50) | 9.4 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [4] |
| Half Maximal Inhibitory Concentration (IC50) | 9.5 | nM |
Peripheral blood mononuclear cells
|
Normal
|
Undisclosed | [47] |
| Half Maximal Inhibitory Concentration (IC50) | 920 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[101] | |
| Half Maximal Inhibitory Concentration (IC50) | 9400 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[33] | |
| Half Maximal Inhibitory Concentration (IC50) | 9500 | nM |
BV-2 cells
|
Normal
|
[102] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 95060.48 | nM |
MOLT-4 cells
|
Adult T acute lymphoblastic leukemia
|
[25] | |
| Half Maximal Cell Growth Inhibitory Concentration (GI50) | 95940.06 | nM |
DMS 114 cells
|
Lung small cell carcinoma
|
[25] | |
| Half Maximal Inhibitory Concentration (IC50) | 980 | nM |
RAW264.7 cells
|
Monocytic-macrophage leukemia
|
[103] |
Each Antibody-drug Conjugate Related to This Payload
References
