Linker Information
General Information of This Linker
| Linker ID |
LIN0ZEYRW
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Pyrimidine-CL2A-carbonate
|
|||||
| Linker Type |
pH-sensitive linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
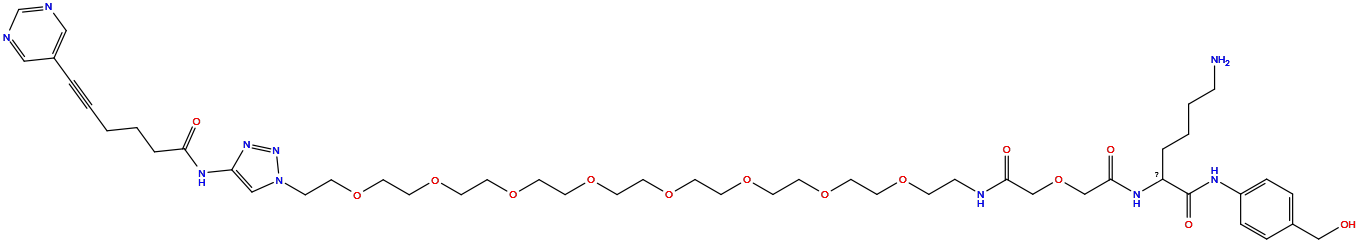
|
|||||
| Formula |
C47H72N10O14
|
|||||
| Isosmiles |
NCCCCC(NC(=O)COCC(=O)NCCOCCOCCOCCOCCOCCOCCOCCOCCn1cc(NC(=O)CCCC#Cc2cncnc2)nn1)C(=O)Nc1ccc(CO)cc1
|
|||||
| InChI |
InChI=1S/C47H72N10O14/c48-13-5-4-7-42(47(62)52-41-11-9-39(35-58)10-12-41)53-46(61)37-71-36-45(60)51-14-16-63-18-20-65-22-24-67-26-28-69-30-31-70-29-27-68-25-23-66-21-19-64-17-15-57-34-43(55-56-57)54-44(59)8-3-1-2-6-40-32-49-38-50-33-40/h9-12,32-34,38,42,58H,1,3-5,7-8,13-31,35-37,48H2,(H,51,60)(H,52,62)(H,53,61)(H,54,59)
|
|||||
| InChIKey |
JUDMUQGUSXPCEE-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
1001.149
|
Polar area
|
302.21
|
||
|
Complexity
|
1000.522947
|
xlogp Value
|
0.239
|
|||
|
Heavy Count
|
71
|
Rot Bonds
|
43
|
|||
|
Hbond acc
|
20
|
Hbond Donor
|
6
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Sacituzumab tirumotecan [Approved]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05347134 | Clinical Status | Phase 3 | ||
| Clinical Description |
A randomized, controlled, open-label, multi-center phase 3 clinical trial of SKB264 for injection versus investigator selected regimens in patients with unresectable locally advanced, recurrent or metastatic triple-negative breast cancer who have failed second-line or above prior standard of care.
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05642780 | Clinical Status | Phase 2 | ||
| Clinical Description |
Amulticenter, open-label, phase 2, basket study to evaluate the efficacy and safety of SKB264 in combination with pembrolizumab in subjects with selected solid tumors.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05631262 | Clinical Status | Phase 2 | ||
| Clinical Description |
A multicenter, open-label, phase 2 study to evaluate the efficacy and safety of SKB264 monotherapy in selected subjects with advanced solid tumors.
|
||||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05445908 | Clinical Status | Phase 2 | ||
| Clinical Description |
A phase 2 clinical study of SKB264 with/without KL-A167 in patients with unresectable locally advanced, recurrent or metastatic triple-negative breast cancer (TNBC) who have not received prior systemic therapy.
|
||||
| Experiment 5 Reporting the Activity Date of This ADC | [5] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT05351788 | Clinical Status | Phase 2 | ||
| Clinical Description |
A phase 2 clinical study of combination therapy of SKB264 in patients with advanced or metastatic non-small cell lung cancer.
|
||||
Discovered Using Patient-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 30.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 30.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 38.40% (Day 21) | Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 4 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.90% (Day 21) | Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 5 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
44.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 0.5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | < 50.00% (Day 21) | Negative TROP2 expression (TROP2-) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 7 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 66.70% (Day 21) | High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 8 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
92.60% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 1.5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
| Experiment 9 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 10 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 11 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 10 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 12 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 406022) | ||||
| Experiment 13 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Moderate TROP2 expression (TROP2++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX model (PDX: A11068) | ||||
| Experiment 14 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 21)
|
Low TROP2 expression (TROP2+) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 3 mg/kg for gastric cancer PDX models twice a week for six times.
|
||||
| In Vivo Model | Gastric cancer PDX models (PDX: 0501116) | ||||
| Experiment 15 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
The tumor-bearing mice were treated with SKB264 via i.v. injection at doses of 5 mg/kg for BR1282 PDX models twice a week for six times.
|
||||
| In Vivo Model | Breast cancer PDX model (PDX: BR1282) | ||||
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
51.20% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 3 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
75.60% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 1 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
78.40% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 0.3 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
98.50% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 3 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 10 mg/kg in the HCC1806.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) |
100.00% (Day 24)
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
When the average tumor volume reached about 120 mm3, mice were randomized into five groups (n = 8) and subsequently administered by intravenous (i.v.) injection with the testing item twice a week for six times. SKB264 treatments were performed at doses of 1 mg/kg in the NCI-N87.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.28 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations:Calu-3 (8,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | Calu-3 cells | CVCL_0609 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.24 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCI-N87 (5,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
3.41 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCI-H23 (TROP2+, 3,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H23 cells | CVCL_1547 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
5.70 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: HCC1806 (3,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Breast squamous cell carcinoma | HCC1806 cells | CVCL_1258 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
11.03 nM
|
High TROP2 expression (TROP2+++) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: BxPC-3 (2,000 cells per well),After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [6] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.83 nM
|
Negative TROP2 expression (TROP2-) | ||
| Method Description |
Tumor cells were seeded on 96-well plates at the following concentrations: NCIH23 (parental, 3,000 cells per well).After overnight incubation,the diluted testing items were added respectively. After 72 h, cell viability was evaluated using a CellTiter-Glo Luminescent Cell Viability Assay.
|
||||
| In Vitro Model | Lung adenocarcinoma | NCI-H23 cells | CVCL_1547 | ||
References
