Linker Information
General Information of This Linker
| Linker ID |
LIN0RTVRG
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
CL2E
|
|||||
| Linker Type |
pH-sensitive linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
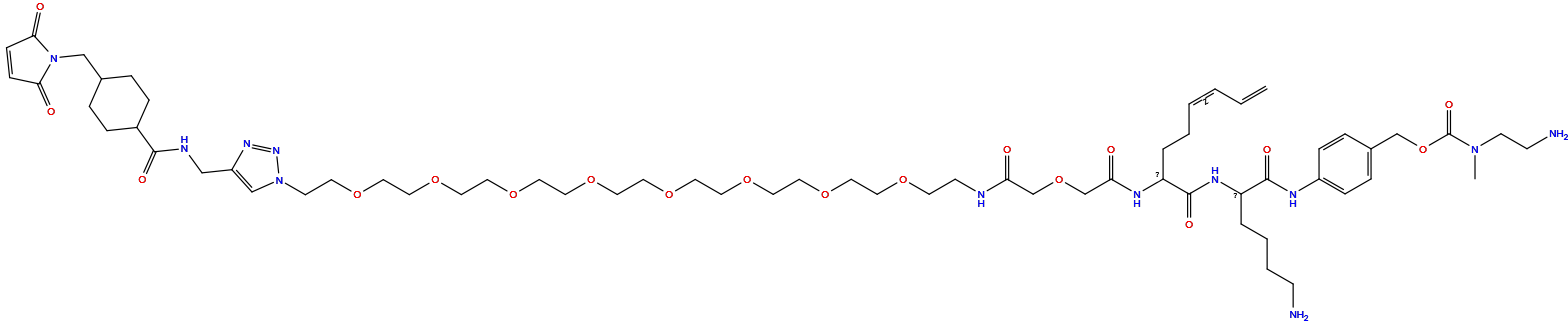
|
|||||
| Formula |
C62H98N12O18
|
|||||
| Isosmiles |
C=C/C=C\CCC(NC(=O)COCC(=O)NCCOCCOCCOCCOCCOCCOCCOCCOCCn1cc(CNC(=O)C2CCC(CN3C(=O)C=CC3=O)CC2)nn1)C(=O)NC(CCCCN)C(=O)Nc1ccc(COC(=O)N(C)CCN)cc1
|
|||||
| InChI |
InChI=1S/C62H98N12O18/c1-3-4-5-6-9-53(61(81)69-54(10-7-8-21-63)60(80)67-51-17-13-49(14-18-51)45-92-62(82)72(2)24-22-64)68-56(76)47-91-46-55(75)65-23-26-83-28-30-85-32-34-87-36-38-89-40-41-90-39-37-88-35-33-86-31-29-84-27-25-73-44-52(70-71-73)42-66-59(79)50-15-11-48(12-16-50)43-74-57(77)19-20-58(74)78/h3-5,13-14,17-20,44,48,50,53-54H,1,6-12,15-16,21-43,45-47,63-64H2,2H3,(H,65,75)(H,66,79)(H,67,80)(H,68,76)(H,69,81)/b5-4-
|
|||||
| InChIKey |
BLGPAMLUUFJKRY-PLNGDYQASA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
1299.532
|
Polar area
|
378.24
|
||
|
Complexity
|
92
|
xlogp Value
|
0.6781
|
|||
|
Heavy Count
|
92
|
Rot Bonds
|
53
|
|||
|
Hbond acc
|
23
|
Hbond Donor
|
7
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Epratuzumab-CL2E-SN-38 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
42 Day
|
Low CD22 expression (CD22+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.15 mg/dose (2.4 g SN-38 equivalents).
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
63 Day
|
Low CD22 expression (CD22+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.30 mg/dose (4.8 g SN-38 equivalents).
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
140 Day
|
Low CD22 expression (CD22+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.15 mg/dose (2.4 g SN-38 equivalents) plus Veltuzumab, 35 ug/dose.
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) | > 161 Day | Low CD22 expression (CD22+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.30 mg/dose (4.8 g SN-38 equivalents) plus Veltuzumab, 35 ug/dose.
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.06 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.19 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.20 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.34 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.38 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with SN38 and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.41 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.46 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.46 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.50 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with SN38 and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 10 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.51 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 11 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.52 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 12 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.68 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 13 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.73 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 14 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.81 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 15 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.83 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 16 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.84 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 17 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.16 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 18 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.16 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with veltuzumab (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 19 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.22 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 20 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.50 nM
|
High CD22 expression (CD22+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 21 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.66 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 22 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.68 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Adult B acute lymphoblastic leukemia | RS4;11 cells | CVCL_0093 | ||
| Experiment 23 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.72 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with SN38 and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 24 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.77 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with SN38 and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 25 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.84 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with SN38 and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 26 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | . 2.10 nM | Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 27 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.21 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 28 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.25 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 29 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.29 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 30 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.45 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (1.33 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 31 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.67 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Childhood B acute lymphoblastic leukemia | 697 cells | CVCL_0079 | ||
| Experiment 32 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.88 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay. Cells were co-incubated with hRS7 (133 nmol/L) and increasing concentrations of Emab-SN-38.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 33 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.92 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 34 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
3.65 nM
|
Low CD22 expression (CD22+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | MN-60 cells | CVCL_1421 | ||
| Experiment 35 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
135.80 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 36 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
152.30 nM
|
Moderate CD22 expression (CD22++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 37 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
271 nM
|
High CD20 expression (CD20+++) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Labetuzumab-CL2E-SN-38 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
63 Day
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.30 mg/dose (4.8 g SN-38 equivalents).
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
76 Day
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.15 mg/dose (2.4 g SN-38 equivalents).
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
91 Day
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.30 mg/dose (4.8 g SN-38 equivalents) plus Veltuzumab, 35 ug/dose.
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Median survival time (MST) |
98 Day
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
The intravenous WSU-FSCCL models were initiated by intravenous injection of 2.5 x 106 cells, in female severe combined immunodeficient (SCID) mice (Taconic). The dose was 0.15 mg/dose (2.4 g SN-38 equivalents) plus Veltuzumab, 35 ug/dose.
|
||||
| In Vivo Model | WSU-FSCCL CDX model | ||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.17 nM
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Mantle cell lymphoma | JeKo-1 cells | CVCL_1865 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
3.73 nM
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
8.08 nM
|
Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Daudi cells | CVCL_0008 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Negative CEACAM5 expression (CEACAM5-) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Low CEACAM5 expression (CEACAM5+) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Follicular lymphoma | WSU-FSCCL cells | CVCL_1903 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Negative CEACAM5 expression (CEACAM5-) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 7 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Negative CEACAM5 expression (CEACAM5-) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Childhood B acute lymphoblastic leukemia | 697 cells | CVCL_0079 | ||
| Experiment 8 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Negative CEACAM5 expression (CEACAM5-) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Adult B acute lymphoblastic leukemia | RS4;11 cells | CVCL_0093 | ||
| Experiment 9 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 50.00 nM | Negative CEACAM5 expression (CEACAM5-) | ||
| Method Description |
Cytotoxicity was determined using the MTS dye reduction assay.
|
||||
| In Vitro Model | Burkitt lymphoma | MN-60 cells | CVCL_1421 | ||
Lmab-CL2A-SN38 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
1.70 nM
|
|||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
2.80 nM
|
|||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
5.10 nM
|
|||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
Emab-CL2E-SN38 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
77.90 nM
|
Low CD22 expression (CD22+; Median fluorescence=22.9) | ||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | B acute lymphoblastic leukemia | Reh cells | CVCL_1650 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
135.80 nM
|
Moderate CD22 expression (CD22++; Median fluorescence=45.9) | ||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | EBV-related Burkitt lymphoma | Raji cells | CVCL_0511 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
152.30 nM
|
Moderate CD22 expression (CD22++; Median fluorescence=40.8) | ||
| Method Description |
In vitro cytotoxicity by MTS assay of SN-38 and specific ADC conjugates against several hematopoietic tumor cell lines. The effect of linkage stability on the cytotoxicity of antibody conjugates as determined by a 4-day MTS assay.
|
||||
| In Vitro Model | Burkitt lymphoma | Ramos cells | CVCL_0597 | ||
References
