Linker Information
General Information of This Linker
| Linker ID |
LIN0QVNCR
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Man-beta-1,4-GlcNAc-DBCO-PEG5-VC-PAB
|
|||||
| Linker Type |
Sugar-based site-specific conjugation linker; Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
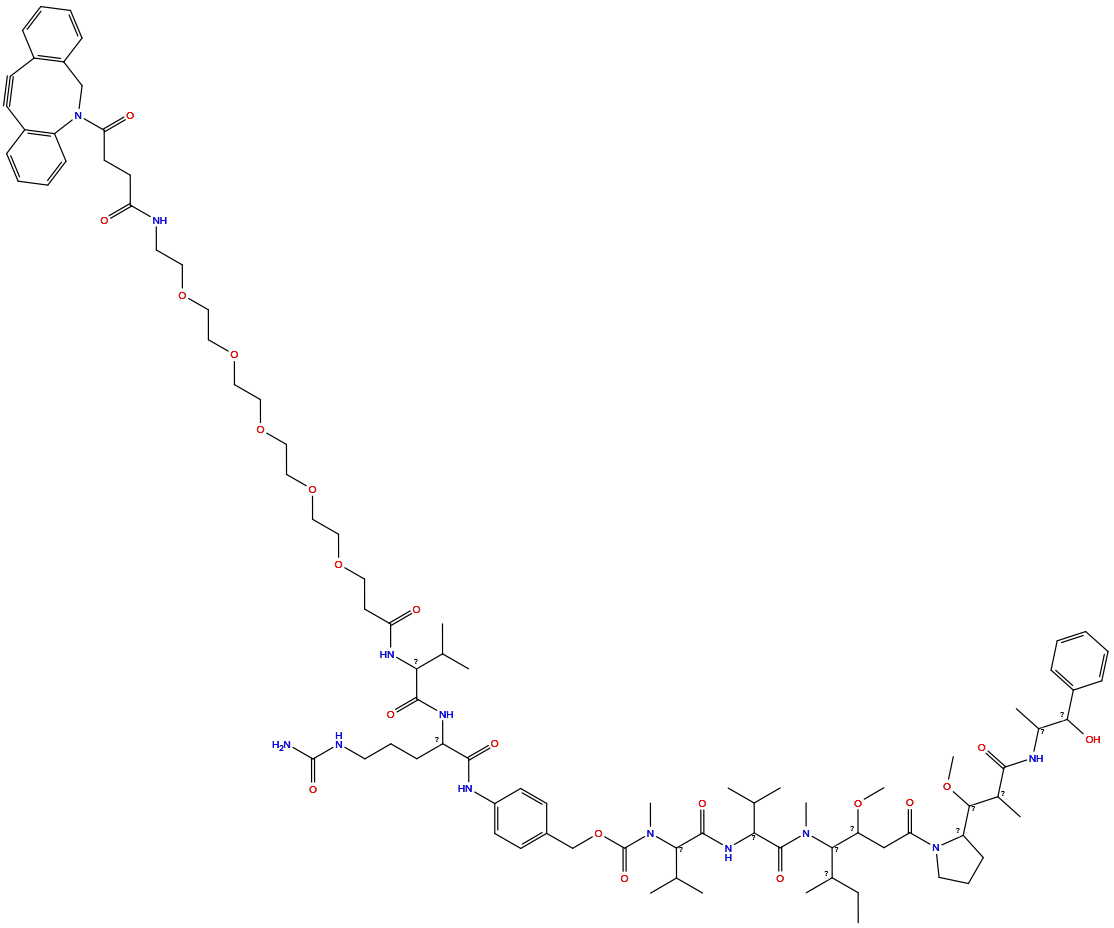
|
|||||
| Formula |
C90H132N12O20
|
|||||
| Isosmiles |
CCC(C)C(C(CC(=O)N1CCCC1C(OC)C(C)C(=O)NC(C)C(O)c1ccccc1)OC)N(C)C(=O)C(NC(=O)C(C(C)C)N(C)C(=O)OCc1ccc(NC(=O)C(CCCNC(N)=O)NC(=O)C(NC(=O)CCOCCOCCOCCOCCOCCNC(=O)CCC(=O)N2Cc3ccccc3C#Cc3ccccc32)C(C)C)cc1)C(C)C
|
|||||
| InChI |
InChI=1S/C90H132N12O20/c1-15-61(8)81(73(115-13)55-77(106)101-44-24-32-72(101)83(116-14)62(9)84(108)94-63(10)82(107)67-27-17-16-18-28-67)99(11)88(112)79(59(4)5)98-87(111)80(60(6)7)100(12)90(114)122-57-64-33-37-69(38-34-64)95-85(109)70(30-23-42-93-89(91)113)96-86(110)78(58(2)3)97-75(104)41-45-117-47-49-119-51-53-121-54-52-120-50-48-118-46-43-92-74(103)39-40-76(105)102-56-68-29-20-19-25-65(68)35-36-66-26-21-22-31-71(66)102/h16-22,25-29,31,33-34,37-38,58-63,70,72-73,78-83,107H,15,23-24,30,32,39-57H2,1-14H3,(H,92,103)(H,94,108)(H,95,109)(H,96,110)(H,97,104)(H,98,111)(H3,91,93,113)
|
|||||
| InChIKey |
WLDJBWZAWMRUNJ-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
1702.11
|
Polar area
|
405.03
|
||
|
Complexity
|
122
|
xlogp Value
|
6.9077
|
|||
|
Heavy Count
|
122
|
Rot Bonds
|
53
|
|||
|
Hbond acc
|
20
|
Hbond Donor
|
9
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Trastuzumab-MMAE conjugate DAR12 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 uM | Negative HER2 expression (HER2-) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.04 nM
7.13 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Trastuzumab-MMAE conjugate DAR8 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 uM | Negative HER2 expression (HER2-) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.05 nM
8.79 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Trastuzumab-MMAE conjugate DAR6 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 uM | Negative HER2 expression (HER2-) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.08 nM
12.68 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Trastuzumab-MMAE conjugate DAR4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 uM | Negative HER2 expression (HER2-) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.10 nM
16.05 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
Trastuzumab-MMAE conjugate DAR2 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) | > 10.00 uM | Negative HER2 expression (HER2-) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines SK-BR-3 and BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | T-47D cells | CVCL_0553 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.07 nM
10.67 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines SK-BR-3 and BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
0.41 nM
6.20 ng/mL |
High HER2 expression (HER2+++) | ||
| Method Description |
The cytotoxicity of the synthetic ADCs were tested in breast cancer cell lines SK-BR-3 and BT474 that have high levels of HER2 expression, and T47D that has low level expression of HER2 antigen.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
References
