Linker Information
General Information of This Linker
| Linker ID |
LIN0OXUQL
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Mc-Val-Ala-PABC
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
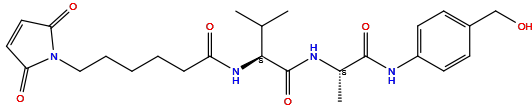
|
|||||
| Formula |
C25H34N4O6
|
|||||
| Isosmiles |
C[C@@H](C(=O)NC1=CC=C(C=C1)CO)NC(=O)[C@H](C(C)C)NC(=O)CCCCCN2C(=O)C=CC2=O
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C25H34N4O6/c1-16(2)23(28-20(31)7-5-4-6-14-29-21(32)12-13-22(29)33)25(35)26-17(3)24(34)27-19-10-8-18(15-30)9-11-19/h8-13,16-17,23,30H,4-7,14-15H2,1-3H3,(H,26,35)(H,27,34)(H,28,31)/t17-,23-/m0/s1
|
|||||
| InChIKey |
DAMSURYLURICHN-SBUREZEXSA-N
|
|||||
| IUPAC Name |
6-(2,5-dioxopyrrol-1-yl)-N-[(2S)-1-[[(2S)-1-[4-(hydroxymethyl)anilino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]hexanamide
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
486.6
|
Polar area
|
145
|
||
|
Complexity
|
786
|
xlogp Value
|
0.9
|
|||
|
Heavy Count
|
35
|
Rot Bonds
|
13
|
|||
|
Hbond acc
|
6
|
Hbond Donor
|
4
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Tamrintamab pamozirine [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Partial Response (PR) |
1.72%
|
|||
| Patients Enrolled |
Female patients (age 18 years) with EOC if they had evidence of progressive disease during or within 6 months of receiving a platinum regimen.
|
||||
| Administration Dosage |
1 of 6 dose levels (0.025-0.40 mg/kg) every 3 weeks (Q3W), utilizing a standard 3+3 design (dose-limiting toxicity [DLT] period: 21 days).
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02539719 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1a/1b dose escalation and expansion study of SC-003 as a single-agent and in combination with ABBV-181 in subjects with platinum-resistant/ refractory ovarian cancer.
|
||||
| Primary Endpoint |
The MTD for the Q3W schedule was 0.30 mg/kg and the SC-003 doses selected for the dose-expansion phase of the study were 0.30 mg/kg and 0.20 mg/kg.
|
||||
| Other Endpoint |
ORR=5.17% (N=3/58), 3 patients achieved PR. All responses were observed at 0.20-0.30mg/kg. Responses were not durable, with only 1 PR confirmed on the follow-up 16-week scan.
|
||||
Cetuximab- (FGX16-11) [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 42.79% (Day 36) | High EGFR expression (EGFR+++) | ||
| Method Description |
The inhibitory activity of Cetuximab-(FGX16-11) against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 1 mg/kg.
|
||||
| In Vivo Model | Colon cancer CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 63.01% (Day 36) | High EGFR expression (EGFR+++) | ||
| Method Description |
The inhibitory activity of Cetuximab-(FGX16-11) against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 5 mg/kg.
|
||||
| In Vivo Model | Colon cancer CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 92.22% (Day 36) | High EGFR expression (EGFR+++) | ||
| Method Description |
The inhibitory activity of Cetuximab-(FGX16-11) against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 10 mg/kg.
|
||||
| In Vivo Model | Colon cancer CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 99.98% (Day 36) | High EGFR expression (EGFR+++) | ||
| Method Description |
The inhibitory activity of Cetuximab-(FGX16-11) against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 20 mg/kg.
|
||||
| In Vivo Model | Colon cancer CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 36) | High EGFR expression (EGFR+++) | ||
| Method Description |
The inhibitory activity of Cetuximab-(FGX16-11) against cancer cell growth was evaluated in various human cancer cell lines in vivo. The cells were treated with 40 mg/kg.
|
||||
| In Vivo Model | Colon cancer CDX model | ||||
| In Vitro Model | Colon adenocarcinoma | SW48 cells | CVCL_1724 | ||
Anti-HER2-D265C-30.2371 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
8.00 pM
|
Positive HER2 expression (HER2+++/++; HER2 MFI=1016) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
10.00 pM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
71.00 pM
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Anti-PSMA-D265C-30.2371 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.00 pM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
71.00 pM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Anti-HER2-D265C-30.1699 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
17.00 pM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
18.00 pM
|
Positive HER2 expression (HER2+++/++; HER2 MFI=1016) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11 nM
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Anti-PSMA-D265C-30.1699 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
22.00 pM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.11 nM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Anti-HER2-D265C-30.2115 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
25.00 pM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
54.00 pM
|
Positive HER2 expression (HER2+++/++; HER2 MFI=1016) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.28 nM
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Anti-HER2-D265C-30.2060 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
31.00 pM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
32.00 pM
|
Positive HER2 expression (HER2+++/++; HER2 MFI=1016) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.21 nM
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Anti-PSMA-D265C-30.2060 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
32.00 pM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.19 nM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Anti-PSMA-D265C-30.2347 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
43.00 pM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.16 nM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Anti-HER2-D265C-30.2347 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
46.00 pM
|
Positive HER2 expression (HER2+++/++; HER2 MFI=1016) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
59.00 pM
|
Positive HER2 expression (HER2 +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.13 nM
|
Moderate HER2 expression (HER2++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-HER2 antibody carrying a D265C mutation (T-D265C, anti-HER2-D265C) conjugated tostructurally different amanitin derivatives via its D265C residue was tested on JIMT-1 cells NCI-N87 cells and SKBR-3 cells.
|
||||
| In Vitro Model | Breast ductal carcinoma | JIMT-1 cells | CVCL_2077 | ||
Anti-PSMA-D265C-30.2115 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.27 nM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | LNCaP cells | CVCL_0395 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [3] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.36 nM
|
Positive PSMA expression (PSMA +++/++) | ||
| Method Description |
The cytotoxic activity in vitro of ADCs, which are comprising an anti-PSMA antibody carrying a D265C mutation conjugated tostructurally different amanitin derivatives via its D265C residue was tested on LNCaP cells and 22RV1 cells.
|
||||
| In Vitro Model | Prostate carcinoma | 22RV1 cells | CVCL_1045 | ||
Mil40-5 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
14.50 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Invasive breast carcinoma | BT474 HerDR cells | CVCL_0179 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
157.60 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | Negative HER2 expression (HER2-) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [4] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 1000.00 nM | Negative HER2 expression (HER2-) | ||
| Method Description |
SKOV-3, BT474 HerDR, MDA-MB-231 and MCF-7 cells were cultured under various concentrations of Mil40, SN-38 and ADCs for 10days, 9days, 6days, and 6days, respectively. Cytotoxicity assays were established using the CellTiter-Go assay kit (CTG).
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
PD-L1 ADC 1 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
265.30 nM
|
High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 1 and ADC 2 was evaluated in three PD-L1-positive cell lines, i.e., Calu-1, MDA-MB-231, and SK-MES, and one PD-L1-negative cell line, i.e., AsPC-1.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | SK-MES-1 cells | CVCL_0630 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 663.08 nM | High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 1 and ADC 2 was evaluated in three PD-L1-positive cell lines, i.e., Calu-1, MDA-MB-231, and SK-MES, and one PD-L1-negative cell line, i.e., AsPC-1.
|
||||
| In Vitro Model | Lung squamous cell carcinoma | Calu-1 cells | CVCL_0608 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 663.08 nM | High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 1 and ADC 2 was evaluated in three PD-L1-positive cell lines, i.e., Calu-1, MDA-MB-231, and SK-MES, and one PD-L1-negative cell line, i.e., AsPC-1.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 663.08 nM | Negative PD-L1 expression (PD-L1-) | ||
| Method Description |
The in vitro cytotoxicity of ADC 1 and ADC 2 was evaluated in three PD-L1-positive cell lines, i.e., Calu-1, MDA-MB-231, and SK-MES, and one PD-L1-negative cell line, i.e., AsPC-1.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | AsPC-1 cells | CVCL_0152 | ||
PD-L1 ADC 3 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
9.75 nM
|
High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 3 was quickly evaluated in three PD-L1-positive cell lines, ie, MDA-MB-231, PC 9, and A431, and one PD-L1-negative cell line, ie, Romas.
|
||||
| In Vitro Model | Lung adenocarcinoma | PC-9 cells | CVCL_B260 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
10.33 nM
|
High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 3 was quickly evaluated in three PD-L1-positive cell lines, ie, MDA-MB-231, PC 9, and A431, and one PD-L1-negative cell line, ie, Romas.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-231 cells | CVCL_0062 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [5] | ||||
| Efficacy Data | Half Maximal Effective Concentration (EC50) |
11.94 nM
|
High PD-L1 expression (PD-L1+++) | ||
| Method Description |
The in vitro cytotoxicity of ADC 3 was quickly evaluated in three PD-L1-positive cell lines, ie, MDA-MB-231, PC 9, and A431, and one PD-L1-negative cell line, ie, Romas.
|
||||
| In Vitro Model | Skin squamous cell carcinoma | A431 cells | CVCL_0037 | ||
References
