Linker Information
General Information of This Linker
| Linker ID |
LIN0NUGJD
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Open-chain glycine maleimide
|
|||||
| Linker Type |
Flexible reactive (thiol) linker
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
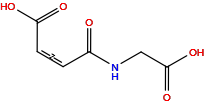
|
|||||
| Formula |
C6H7NO5
|
|||||
| Isosmiles |
O=C(O)/C=C\C(=O)NCC(=O)O
|
|||||
| InChI |
InChI=1S/C6H7NO5/c8-4(1-2-5(9)10)7-3-6(11)12/h1-2H,3H2,(H,7,8)(H,9,10)(H,11,12)/b2-1-
|
|||||
| InChIKey |
JKCBZBFTTXFZGH-UPHRSURJSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
173.124
|
Polar area
|
103.7
|
||
|
Complexity
|
12
|
xlogp Value
|
-1.172
|
|||
|
Heavy Count
|
12
|
Rot Bonds
|
4
|
|||
|
Hbond acc
|
3
|
Hbond Donor
|
3
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
C4.4A-EC4 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 60.70% (Day 80) | High LYPD3 expression (LYPD3+++) | ||
| Method Description |
The in vivo antitumor efficacy and tolerability of the selected NAMPTi-ADCs were evaluated in the cell line-derived MDA-MB-453 human breast cancer xenograft models. Treatment with the C4.4a-EC4 (10 mg/kg,iv,Q7Dx7).
|
||||
| In Vivo Model | Breast cancer CDX model | ||||
| In Vitro Model | Breast cancer | Breast cancer cells | Homo sapiens | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.30 nM
|
High CD276 expression (CD276+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | A549-C4.4a cells | CVCL_0023 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
58.00 nM
|
High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
HER2-EC4 DAR7.8 [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 88.10% (Day 80) | High LYPD3 expression (LYPD3+++) | ||
| Method Description |
The in vivo antitumor efficacy and tolerability of the selected NAMPTi-ADCs were evaluated in the cell line-derived MDA-MB-453 human breast cancer xenograft models. Treatment with the HER2-EC4 (10 mg/kg,iv,Q7Dx7).
|
||||
| In Vivo Model | Breast caner CDX model | ||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 pM
|
High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
300.00 nM
|
High CD276 expression (CD276+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | A549-C4.4a cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
Anti-B7H3 APDC 3f [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 90.04% (Day 30) | Positive B7H3 expression (B7H3 +++/++) | ||
| Method Description |
Both APDCs (10 mg/kg) have been administered to B7H3-positive U251-glioma-tumor-bearing mice; 24hours after treatment, tumors and organs were collected.
|
||||
| In Vivo Model | U251 CDX model | ||||
| In Vitro Model | Astrocytoma | U-251MG cells | CVCL_0021 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 100.00% (Day 50) | Positive B7H3 expression (B7H3 +++/++) | ||
| Method Description |
In the B7H3-positive, slowly growing head and neck squamous cell carcinoma model SCC4, APDC 3 g at a dose of 10 mg/kg given 3 times in a weekly schedule which favorably compared to cisplatin and radiation therapy.
|
||||
| In Vivo Model | SCC4 CDX model | ||||
| In Vitro Model | Tongue squamous cell carcinoma | SCC-4 cells | CVCL_1684 | ||
Anti-B7H3 APDC 3g [Investigative]
Discovered Using Cell Line-derived Xenograft Model
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Tumor Growth Inhibition value (TGI) | ≈ 94.08% (Day 30) | Positive B7H3 expression (B7H3 +++/++) | ||
| Method Description |
Both APDCs (10 mg/kg) have been administered to B7H3-positive U251-glioma-tumor-bearing mice; 24hours after treatment, tumors and organs were collected.
|
||||
| In Vivo Model | U251 CDX model | ||||
| In Vitro Model | Astrocytoma | U-251MG cells | CVCL_0021 | ||
B7H3-EC4 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | < 0.00 nM | High CD276 expression (CD276+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 300.00 nM | High LYPD3 expression (LYPD3+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | A549-C4.4a cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
HER2-EC4 DAR2.7 [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.04 nM
|
High CD276 expression (CD276+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-453 cells | CVCL_0418 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
300.00 nM
|
High LYPD3 expression (LYPD3+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Lung adenocarcinoma | A549-C4.4a cells | CVCL_0023 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) | > 10.00 uM | High LYPD3 expression (LYPD3+++); High HER2 expression (HER2+++) | ||
| Method Description |
The in vitro potency of NAMPTi-SMOLs and NAMPTi-ADCs was determined in human tumor cell lines. Cells (2000-4000 cells/well, were incubated at 37°C, 5% CO2 for 24 h and the compounds were added at concentrations of 3x10-12 - 3x10-8 Min triplicates. Cell viability was determined at the beginning (day 0) and after 72-96 h incubation in the presence or absence of NAMPTi-SMOLs or NAMPTi-ADCs.
Click to Show/Hide
|
||||
| In Vitro Model | Childhood acute monocytic leukemia | THP-1 cells | CVCL_0006 | ||
TWEAKR ADC 3a [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.10 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.90 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3g [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.20 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3f [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.70 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.90 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3e [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.90 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.20 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3c [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.30 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.80 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
2.10 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3b [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.60 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.60 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
1.10 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
TWEAKR ADC 3d [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
600 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Lung mucoepidermoid carcinoma | NCI-H292 cells | CVCL_0455 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
600 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Pancreatic ductal adenocarcinoma | BxPC-3 cells | CVCL_0186 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [2] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
600 nM
|
Positive TNFRSF12A expression (TNFRSF12A +++/++) | ||
| Method Description |
Cytotoxicity of ADCs, SMOL KSP inhibitors, and payload metabolite were determined in TWEAKR-positive cancer cell lines 72 h after treatment with the Promega CellTiter Glo (CTG) cell viability assay according to manufacturers instructions.
|
||||
| In Vitro Model | Colon adenocarcinoma | LoVo cells | CVCL_0399 | ||
References
