Linker Information
General Information of This Linker
| Linker ID |
LIN0KCPHV
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
PEG3-Val-Cit-PABC
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
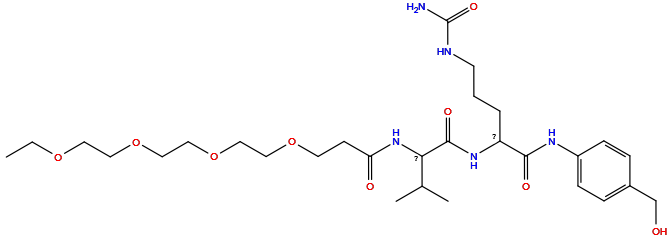
|
|||||
| Formula |
C27H45N5O7
|
|||||
| Isosmiles |
C=C(N[C@@H](CCCNC(N)=O)C(=O)Nc1ccc(CO)cc1)[C@@H](NC(=O)COCCOCCOCC)C(C)C
|
|||||
| InChI |
InChI=1S/C27H45N5O7/c1-5-37-13-14-38-15-16-39-18-24(34)32-25(19(2)3)20(4)30-23(7-6-12-29-27(28)36)26(35)31-22-10-8-21(17-33)9-11-22/h8-11,19,23,25,30,33H,4-7,12-18H2,1-3H3,(H,31,35)(H,32,34)(H3,28,29,36)/t23-,25-/m0/s1
|
|||||
| InChIKey |
ZYXLEBHVQKIHRA-ZCYQVOJMSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
551.685
|
Polar area
|
173.27
|
||
|
Complexity
|
551.3318988
|
xlogp Value
|
1.2483
|
|||
|
Heavy Count
|
39
|
Rot Bonds
|
21
|
|||
|
Hbond acc
|
8
|
Hbond Donor
|
6
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
SYSA-1801 [Phase 1 (Terminated)]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
47.10% (gastric cancer)
33.30% (1.00 mg/kg) 40.00% (2.00 mg/kg) 100.00% (2.50 mg/kg) 20.00% (3.00 mg/kg) 38.10% (all) |
|||
| Patients Enrolled |
Patients with resistant/refractory solid tumors that express CLDN18.2 who progressed on or were intolerant to standard treatment, or had no standard treatment were recruited.. ECOG score of 0-2.
|
||||
| Administration Dosage |
0.50 up to 3.00 mg/kg on day 1, administered once every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT05009966 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1 trial to evaluate safety, tolerability, pharmacokinetics, immunogenicity and initial efficacy of SYSA1801 in the treatment of CLDN 18.2 positive advanced malignant solid tumor.
|
||||
References
