Linker Information
General Information of This Linker
| Linker ID |
LIN0GOSDZ
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Tetraxetan
|
|||||
| Linker Type |
Chelating agent
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
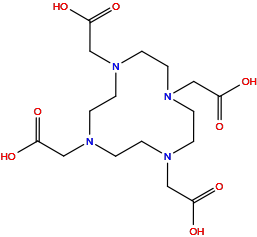
|
|||||
| Formula |
C16H28N4O8
|
|||||
| Isosmiles |
C1CN(CCN(CCN(CCN1CC(=O)O)CC(=O)O)CC(=O)O)CC(=O)O
|
|||||
| PubChem CID | ||||||
| InChI |
InChI=1S/C16H28N4O8/c21-13(22)9-17-1-2-18(10-14(23)24)5-6-20(12-16(27)28)8-7-19(4-3-17)11-15(25)26/h1-12H2,(H,21,22)(H,23,24)(H,25,26)(H,27,28)
|
|||||
| InChIKey |
WDLRUFUQRNWCPK-UHFFFAOYSA-N
|
|||||
| IUPAC Name |
2-[4,7,10-tris(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetic acid
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
404.42
|
Polar area
|
162
|
||
|
Complexity
|
447
|
xlogp Value
|
-10.6
|
|||
|
Heavy Count
|
28
|
Rot Bonds
|
8
|
|||
|
Hbond acc
|
12
|
Hbond Donor
|
4
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Rosopatamab tetraxetan [Investigative]
Identified from the Human Clinical Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Objective Response Rate (ORR) |
9.38% (breast cancer)
0.00% (gastric cancer) |
|||
| Patients Enrolled |
Unresectable, locally advanced or metastatic breast cancer or gastric cancer refractory to standard therapy.
|
||||
| Administration Dosage |
Intravenously over 60 to 90 minutes at 0.05, 0.10, 0.20, 0.30, 0.40, 0.50, 0.60, 0.75, or 0.90 mg/kg every 3 weeks.
|
||||
| Related Clinical Trial | |||||
| NCT Number | NCT02576548 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 multicenter, open-label, dose-escalation, and dose-expansion study to evaluate the safety, pharmacokinetics, immunogencity, and antitumor activity of MEDI4276 in subjects with select HER2-expressing advanced solid tumors.
|
||||
| Primary Endpoint |
In patients with breast cancer,ORR=9.38% (N=3/32),DOR=4.20-10.20 months. In the 0.05 to 0.40 mg/kg cohort,PFS=1.3-2.0 months. In the 0.50 to 0.75 mg/kg cohort,PFS=4.60-15.40 months. median overall survival (OS)=19.10 months (range 0.80-30.60, 95% CI 9.6-NA).
|
||||
| Other Endpoint |
No objective responses in patients with gastric cancer,median PFS=1.80 months (range 0-10.7, 95% CI,1.3-3.0),median OS=6.50 months (range 2.80-16.30, 95% CI, 3.10-16.30).
|
||||
| Experiment 2 Reporting the Activity Date of This ADC | [2] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00070837 | Clinical Status | Phase 1 | ||
| Clinical Description |
A phase 1/2 dose escalation trial of multiple doses of mLN2704 (DM1 conjugated monoclonal antibody mLN591) in subjects with metastatic androgen-independent prostate cancer.
|
||||
| Experiment 3 Reporting the Activity Date of This ADC | [3] | ||||
| Related Clinical Trial | |||||
| NCT Number | NCT00052000 | Clinical Status | Phase 1 | ||
| Clinical Description |
Phase 1 dose-escalation study of intravenous CMD-193 in subjects with advanced malignant solid tumors.
|
||||
References
