Linker Information
General Information of This Linker
| Linker ID |
LIN0BUDEO
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
Maleamic methyl ester-based linker 12A
|
|||||
| Linker Type |
Cathepsin-cleavable linker
|
|||||
| Antibody-Linker Relation |
Cleavable
|
|||||
| Structure |
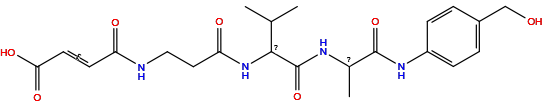
|
|||||
| Formula |
C22H30N4O7
|
|||||
| Isosmiles |
CC(NC(=O)C(NC(=O)CCNC(=O)/C=C/C(=O)O)C(C)C)C(=O)Nc1ccc(CO)cc1
|
|||||
| InChI |
InChI=1S/C22H30N4O7/c1-13(2)20(26-18(29)10-11-23-17(28)8-9-19(30)31)22(33)24-14(3)21(32)25-16-6-4-15(12-27)5-7-16/h4-9,13-14,20,27H,10-12H2,1-3H3,(H,23,28)(H,24,33)(H,25,32)(H,26,29)(H,30,31)/b9-8+
|
|||||
| InChIKey |
TZBMUWBXXWALDK-CMDGGOBGSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
462.503
|
Polar area
|
173.93
|
||
|
Complexity
|
33
|
xlogp Value
|
-0.09
|
|||
|
Heavy Count
|
33
|
Rot Bonds
|
12
|
|||
|
Hbond acc
|
6
|
Hbond Donor
|
6
|
|||
Each Antibody-drug Conjugate Related to This Linker
Full Information of The Activity Data of The ADC(s) Related to This Linker
Mil40-12A [Investigative]
Revealed Based on the Cell Line Data
| Experiment 1 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.02 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3 x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Breast adenocarcinoma | SK-BR-3 cells | CVCL_0033 | ||
| Experiment 2 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.05 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3 x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Gastric tubular adenocarcinoma | NCI-N87 cells | CVCL_1603 | ||
| Experiment 3 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.09 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3 x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Invasive breast carcinoma | BT-474 cells | CVCL_0179 | ||
| Experiment 4 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
0.22 nM
|
High HER2 expression (HER2+++) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3 x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Ovarian serous cystadenocarcinoma | SK-OV-3 cells | CVCL_0532 | ||
| Experiment 5 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
23.31 nM
|
Negative HER2 expression (HER2-) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Invasive breast carcinoma | MCF-7 cells | CVCL_0031 | ||
| Experiment 6 Reporting the Activity Date of This ADC | [1] | ||||
| Efficacy Data | Half Maximal Inhibitory Concentration (IC50) |
24.14 nM
|
Negative HER2 expression (HER2-) | ||
| Method Description |
To evaluate the cytotoxicity, multiple tumor cell lines were treated with three generated maleamic methyl ester-based ADCs. Each group was established three holes, tumor cells (3x104 cells/mL) were added to each well of plate after which 10uL of test compounds solution was added.
|
||||
| In Vitro Model | Breast adenocarcinoma | MDA-MB-468 cells | CVCL_0419 | ||
References
