Linker Information
General Information of This Linker
| Linker ID |
LIN0AUOJQ
|
|||||
|---|---|---|---|---|---|---|
| Linker Name |
AGS-62P1 linker
|
|||||
| Linker Type |
P-acetyl phenylalanine-based site-specific conjugation linker
|
|||||
| Antibody-Linker Relation |
Uncleavable
|
|||||
| Structure |
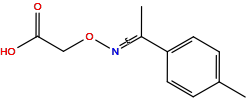
|
|||||
| Formula |
C2H5NO3
|
|||||
| Isosmiles |
NOCC(=O)O
|
|||||
| InChI |
InChI=1S/C2H5NO3/c3-6-1-2(4)5/h1,3H2,(H,4,5)
|
|||||
| InChIKey |
NQRKYASMKDDGHT-UHFFFAOYSA-N
|
|||||
| Pharmaceutical Properties |
Molecule Weight
|
91.066
|
Polar area
|
72.55
|
||
|
Complexity
|
91.02694302
|
xlogp Value
|
-1.0387
|
|||
|
Heavy Count
|
6
|
Rot Bonds
|
2
|
|||
|
Hbond acc
|
3
|
Hbond Donor
|
2
|
|||
Each Antibody-drug Conjugate Related to This Linker
